Consumers are increasingly interested in opalescent or unclarified apple juice. This natural food product provides fibre and nutrients that may be lacking in clarified juices. Opalescent apple juice has lots of pulp, which enhances its sensory properties. However, just like cut apples, this juice turns brown with time. This browning reaction is caused by an enzyme, polyphenol oxidase (PPO). In a process comparable to the rusting of iron, PPO oxidizes the apple's phenolic compounds, which then clump together to form brown pigments. To remedy this problem at the industrial scale, the enzymatic browning reaction is first delayed by adding an antioxidant such as vitamin C. The enzyme is then destroyed using a heat treatment. Although these treatments are effective in terms of preserving colour, they do change the taste of the apple juice.
Achieving balanced acidity
The natural organic acids present in apple juice give it its characteristic slightly acidic taste (pH 3.3 to 3.7). PPO functions very well at this level of acidity. However, by slightly increasing the acidity of the juice, it can be inactivated: simply sprinkling lemon juice (~ pH 2) on cut apple pieces prevents browning. It is therefore possible to inactivate the enzyme by acidifying the juice (down to pH 2). However, in order to obtain a juice that does not make you pucker, the acidity must subsequently be corrected! In the laboratory, the pH can be lowered by adding an acid (such as hydrochloric acid (HCl)) and then raised by adding a base (such as sodium hydroxide or NaOH). When the acid and the base interact, they cancel each other out, producing water (H2O) and salt. However, the salt (in this case, NaCl or table salt) creates a problem. Table salt may be good on tomatoes and cucumbers, but it lends apple juice a quite undesirable salty taste. The solution is bipolar membrane electrodialysis.
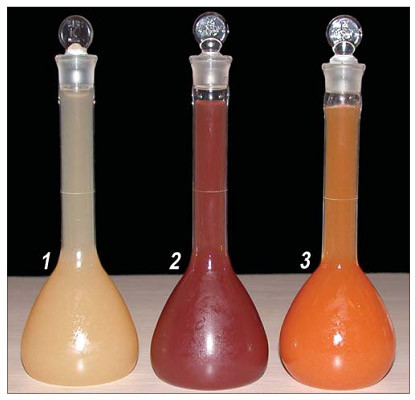
- Sample of opalescent apple juice treated by means of bipolar membrane electrodialysis.
- Sample of PPO-oxidized, untreated opalescent apple juice.
- Sample of commercial or homemade opalescent apple juice.
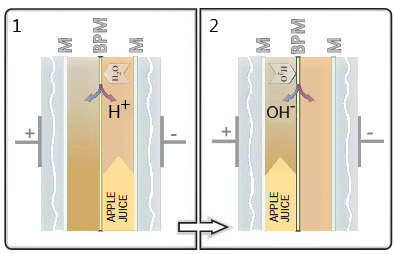
Bipolar membrane (BPM) electrodialysis, in the presence of an electric field, generates H+ and OH− ions from the hydrolysis of the water contained in the juice.
- Circulating the juice in the acid compartment until pH=2.0, and
- Alternating compartments so that the juice returns to its initial pH (3.3 to 3.7) inhibits the enzymatic oxidation reaction in the juice caused by PPO. The ion-selective membrane (M) prevents the juice from being diluted.
This technology uses electricity to literally split the water molecules present in the juice into H+ and OH− ions and direct them into two compartments separated by a membrane: apple juice and the H+ ions on one side, and the OH− ions on the other. As is the case when HCl is added, it is the H+ ions that have the effect of acidifying the apple juice. Once the enzyme has been irreversibly inactivated, it is simply a matter of reversing the compartments and repeating the process, thereby restoring the OH− ions in the juice and reducing the acidity to its original level. The major advantage of this technique is that nothing is added to the apple juice; hence, no salt is formed at the end of the treatment. Provided that this treatment is carried out promptly after extraction, the result is a light-coloured opalescent juice that retains all its healthy nutrients and, especially, tastes great!