March 5, 2015
Office of Audit and Evaluation
Executive summary
In 2009, Agriculture and Agri-Food Canada (AAFC) established the Containment, Biosafety and Biosecurity (CBB) Program which provides researchers with guidance towards achieving appropriate levels of work-site containment associated with handling infectious pathogenic organisms and pests based on Public Health Agency of Canada (PHAC) and the Canadian Food Inspection Agency (CFIA) regulations.
The National Containment, Biosafety and Biosecurity Committee (NCBBC) was established in 2009 to oversee the implementation of the program within AAFC's former Research Branch (now the Science and Technology Branch (STB)) and to provide (among other things) guidance with respect to all aspects of containment, biosafety, and biosecurity.
The Audit of Real Property (Laboratories) was approved by the Deputy Minister in the Agriculture and Agri-Food Canada 2013-2016 Risk-Based Internal Audit Plan for Fiscal Year 2013-2014. The audit covers the period ending December 31, 2013.
The focus of the audit was on the management practices of the AAFC CCB Program for Level 2 laboratories (Containment Level 2 and Plant Pathogen Containment Level 2) within the department.
Research that requires Level 2 containment takes place in a building with secure access and a secure physical area that meets the requirements set by the either CFIA or PHAC. This can be a single room, a series of co-located rooms, or several adjoining rooms of the same containment level.
Containment Level 2 (CL-2) laboratories which handle human and animal pathogens are defined by PHAC as having moderate risk to individuals and low risk to the community. PHAC requires that CL-2 laboratories put in place safeguards to mitigate the risk associated with using Level 2 pathogens that include (among other things) the use of biosafety equipment such as a Biosafety cabinet, restricted access into the laboratories and use of decontamination equipment (for example autoclave).
Plant Pathogen Containment (PPC-2) laboratories that handle plant pests, can in some cases, pose a threat to agricultural production, forests and natural environments if they are inadvertently released from the lab. CFIA requires that safeguards be put in place in PPC-2 laboratories to prevent the inadvertent release of plant pests, these include (among other things) restricted access via an anteroom, ventilation systems with screened exhaust, and on-site decontamination equipment (for example autoclave).
Since 2009, extensive work has been done by the NCBBC and research centres using Level 2 pathogens in the implementation of the CBB program.
The audit was focused on the management control framework (as defined in Annex A) for the CBB program and not identifying specific breaches in containment, biosafety and biosecurity. However, the audit team did not identify specific breaches during the conduct of the audit.
For the areas reviewed, the audit team determined that management practices in place for the CBB program need moderate improvement. As such, audit recommendations were addressed to the Assistant Deputy Minister (ADM), STB for improvements for the following areas:
- Oversight;
- Roles and responsibilities;
- Training; and
- Sharing of lessons learned within the program.
1.0 Introduction
1.1 Background
- 1.1.1 The Human Pathogen Toxin Act (HPTA) received Royal Assent on June 23, 2009 and was enacted to establish a safety and security regime to protect the health and safety of the public against the risks posed by human pathogens and toxins. Public Health Agency of Canada (PHAC) has developed guidelines for the implementation of the HPTA that outline the minimum requirements to safely handle and contain human and terrestrial animal pathogens in a containment laboratory.
- 1.1.2 Human and animal pathogens are designated by PHAC on four levels of risk (Containment Levels (CL) ranging from CL-1 to CL-4 with the latter posing the highest risk) and PHAC has defined corresponding processes and practices required within laboratories for safely handling the various levels of pathogens (See Annex D for additional information).
- 1.1.3 Canada's Plant Protection Act (PPA) was enacted in 2009 and serves to protect plant life and the agricultural and forestry sectors of the Canadian economy by preventing the importation, exportation and spread of pests, and by controlling or eradicating pests in Canada. The PPA gives CFIA the authority to prohibit or restrict the movement of pests into, within, and out of Canada. In response, Canadian Food Inspection Agency (CFIA) developed standards to implement the PPA and describe the minimum acceptable physical and operational requirements for facilities working with plant pests.
- 1.1.4 Plant Pest Containment (PPC) is designated by CFIA on three levels (PPC-1 to PPC-3 with PPC-3 posing the highest risk) with containment achieved through the use of defined facility design, operational procedures and the use of specialized equipment (See Annex E for additional information).
- 1.1.5 Agriculture and Agri-Food Canada's (AAFC) Science and Technology Branch (STB) provides Canada's agriculture, agri-food and agri-based products sectors with research and technology to help them compete in markets at home and abroad. Science and Technology Branch (STB) has approximately 1,530 scientists, technicians and staff who conduct research at a number of centres located across the country.
- 1.1.6 Research at AAFC laboratories includes (among other things) the handling, importation, distribution and field testing of human and/or animal pathogens, as well plant pathogens. Associated with these types of research are containment, biosafety and biosecurity concerns which require effective management to ensure that research is conducted safely and in conformity with relevant Acts, Regulations, Guidelines and Standards.
- 1.1.7 In response to the legislative requirements and the development of PHAC and CFIA guidelines, the AAFC Containment, Biosafety and Biosecurity (CBB) program was established in 2009 to ensure that research projects using pathogens, pests, and other organisms requiring containment (for example biocontrol arthropods, plants, etc.) comply with regulations to reduce the likelihood of the inadvertent release of pathogens and to ensure the safe handling by laboratory staff.
- 1.1.8 The CBB program is responsible for laboratories handling infectious pathogenic organisms and pests and as such, the Level 1 laboratories within the department do not fall under the responsibility of the program as they do not handle infectious pathogens or pests.
- 1.1.9 Agriculture and Agri-Food Canada (AAFC) currently maintains two types of Level 2 containment laboratories, Containment Level 2 (CL-2) for human and animal pathogens and Plant Pest Containment Level 2 (PPC-2) for plant pests in 11 research centres across the country and one PPC-3 laboratory located in the Morden research centre. At the time of this report, the department did not have any Level 4 laboratories. The single Level 3 laboratory at AAFC was not included in the scope of this audit as the laboratory was under construction at the time of the audit.
- 1.1.10 Pathogens used in research activities in a CL-2 lab are defined by PHAC as "any pathogen that can cause human disease but, under normal circumstances, is unlikely to be a serious hazard to laboratory workers, the community, livestock or the environment. Laboratory exposures rarely cause infection leading to serious disease and the risk of spread is limited"Footnote 1. An example of a pathogen that would fall under risk Level 2 for a containment lab would be salmonella.
- 1.1.11 Plant pests used in PPC-2 laboratories almost never infect or infest healthy people, and they therefore pose little direct risk to laboratory personnel. Some can, however, pose a threat to agricultural production, forests and natural environments. As a result, it is important that personnel working with plant pests and the facilities housing these organisms take steps to prevent the accidental escape of potentially damaging pests into the environmentFootnote 2.
- 1.1.12 With the establishment of the CBB program, a program policy and guidelines were developed based on the applicable regulatory body (either PHAC or CFIA) guidelines for the use of Level 2 human/animal pathogens requiring Level 2 containment and plant pests/pathogens/other organisms requiring containment.
- 1.1.13 National oversight of the CBB Program is provided by the National Containment, Biosafety, and Biosecurity Committee (NCBBC) to ensure that the CBB Program responsibilities are discharged properly. The Chair of the NCBBC is appointed to a three-year term and advises the Branch Operating Committee (BOC) on any issues. The other NCBCC members comprise of Biosafety Officers (BSO) and Biocontainment Officers (BCO).
- 1.1.14 As per the CBB program policy, the management of the CBB Program is primarily conducted at local research centres, with the Associate Director, Research, Development and Technology (AD, RDT) accountable for the implementation of the program at research centres that possess or work with Level 2 pathogens and for ensuring compliance with the policy. A Local Containment, Biosafety and Biosecurity Committee (LCBBC) is also established to oversee the local CBB program activities and report to research centre management. The AD, RDT is responsible for appointing a BSO for CL-2 laboratories and/or a BCO for PPC-2 laboratories who are responsible for developing, implementing, evaluating, and administering the AAFC Program and ensuring compliance with the relevant guidelines. (see Annex C for a visual representation of the reporting structure).
- 1.1.15 The Corporate Management Branch (CMB) also has a role in the management of activities at research centres with the Asset Management and Capital Planning Directorate providing Departmental Security Services as well as Integrated Services. Services provided by Integrated Services include, among others, Facilities Management, Occupational Health and Safety, Training Administration and Staffing support.
- 1.1.16 The audit of Real Property (Laboratories) was approved by the Deputy Minister in the Agriculture and Agri-Food Canada Risk-Based Internal Audit Plan for Fiscal Year 2013-2014.
1.2 Audit objective
- 1.2.1 The objective of this audit was to assess the management practices relating to real property (laboratories).
1.3 Audit scope
- 1.3.1 The audit scope assessed the management practicesFootnote 3 of the Agriculture and Agri-Food Canada (AAFC) Containment and Biosafety, Biosecurity (CBB) Program and focused on Level 2 laboratories within the department.
- 1.3.2 The single Level 3 laboratory at AAFC was not included in the scope of this audit as the laboratory was under construction at the time of the audit.
- 1.3.3 The audit conduct phase work included a review of the management practices in place as at December 31, 2013.
1.4 Audit approach
- 1.4.1 The approach and methodology used for the audit was consistent with the Internal Audit (IA) standards as outlined by the Institute of Internal Auditors (IIA), and aligned with the Internal Audit Policy for the Government of Canada (GC).
- 1.4.2 Audit criteria were selected from the Agriculture and Agri-Food Canada (AAFC) Containment, Biosafety, and Biosecurity (CBB) Guidelines, Public Health Agency of Canada's (PHAC) Laboratory Biosafety Guidelines, 3rd edition, and Canadian Food Inspection Agency's (CFIA) Containment Standards for Facilities Handling Plant Pests.
- 1.4.3 A risk-based audit program was developed that defined audit tasks to assess each audit criterion. Audit evidence was gathered through various methods including interviews, observations, site visits to four (4) research centres, documentation review, and analysis.
1.5 Conclusion
- 1.5.1 For the areas reviewed, the audit team determined that management practices in place for the Containment and Biosafety, Biosecurity (CBB) Program require moderate improvement. Recommendations were made by the audit team in the areas of: oversight, roles and responsibilities, training and the sharing of lessons learned within the program. Science and Technology Branch (STB) has agreed to the findings and has developed action plans to address the recommendations.
1.6 Audit context
- 1.6.1 Agriculture and Agri-Food Canada (AAFC) Containment, Biosafety and Biosecurity (CBB) guidelines, developed in 2009, were updated during the audit in April, 2014 and were reviewed as a part of the assessments conducted by the audit team.
- 1.6.2 Steps have been taken by the national CBB committee during the conduct of the audit to strengthen some of the areas identified for improvement.
- 1.6.3 The National Containment, Biosafety and Biosecurity Committee (NCBBC) and Local Containment, Biosafety and Biosecurity Committee (LCBBC) members conduct the roles and responsibilities of the committee on a volunteer basis, in addition to the requirements of their positions.
1.7 Statement of conformance
- 1.7.1 In the professional opinion of the Chief Audit Executive, sufficient and appropriate audit procedures have been conducted and evidence gathered to support the accuracy of the conclusion provided and contained in this report. The conclusion is based on a comparison of the conditions, as they existed at the time, against pre-established audit criteria that were agreed on with management. The conclusion is applicable only to the entity examined.
- 1.7.2 This audit conforms with the Internal Auditing Standards for the Government of Canada, as supported by the results of the quality assurance and improvement program.
2.0 Detailed observations, recommendations and management responses
- 2.0.1 This section presents the key observations, based on the evidence and analysis associated with the audit, and provides recommendations for improvement.
- 2.0.2 Management responses are included and provide:
- An action plan to address each recommendation;
- A lead responsible for implementation of the action plan; and,
- A target date for completion of the implementation of the action plan.
2.1 Oversight
- 2.1.1 The audit expected that Agriculture and Agri-Food Canada (AAFC) management is provided with appropriate (sufficient, complete, timely and accurate) information relating to the AAFC Containment, Biosafety and Biosecurity (CBB) program.
- 2.1.2 The audit team determined that oversight at the national and local levels did exist and the National Containment, Biosafety and Biosecurity Committee (NCBBC) provide general updates to Science and Technology Branch's (STB) Branch Operating Committee on their activities such as keeping research centres informed of their roles and responsibilities, the revised reporting structure for reporting of incidents and advising that the guidelines and Terms of Reference were being updated.
- 2.1.3 For the centres visited during the audit, it was observed that oversight exists to ensure that the following are in place:
- Bio-security plans;
- Procedures to ensure that access to Level 2 laboratories is limited to authorized individuals; and
- Procedures for the coordination and monitoring of the decontamination, disinfection and disposal of infectious materials.
- 2.1.4 While oversight is provided, there were areas of weakness noted. This was indicated through a number of examples, including the following.
National oversight
- 2.1.5 Given that the program was implemented in 2009, we would expect that AAFC research centres would have established CBB programs in place that are in compliance with regulatory and AAFC guidelines. However, the national committee (at the time of the audit) could not provide evidence on the status of program implementation and no formal (or informal) report had been prepared by the NCBBC to STB senior management, on whether program objectives were achieved as defined in the CBB Policy dated 2009.
- 2.1.6 The audit team would have expected to find that the national committee maintained an accurate and up to date listing of all Level 2 laboratories in the department. However, at the time of the audit, the national committee was unable to provide the audit team with an accurate listing. Recent changes to the regulatory guidelines will assist the committee in maintaining an accurate and up to date listing.
- 2.1.7 While a process was developed by the NCBBC for centres to self-assess and report on their individual program, using a template developed by the NCBBC, the audit team determined that the self-assessment process was neither a complete nor a timely method to ensure that AAFC guideline compliance is being achieved.
- 2.1.8 Internal Audit (IA) determined that the self-assessment process is missing key components to ensure complete information is received and reviewed by the national committee. Also, the CBB program was implemented in 2009; however, some centres were not scheduled by the NCBBC to submit program self-assessments until 2015. The IA team also noted that the review of submitted self-assessments was not timely and in some cases taking close to two years to provide feedback to research centres.
- 2.1.9 The program has faced challenges in finding individuals with the skills required to assist in the review of self-assessments. During the conduct of the audit, the NCBBC implemented the practice of inviting Biosafety Officers (BSO) or Biocontainment Officers (BCO) to assist in the review of the self-assessments in an effort to improve the timeliness in providing feedback to research centres.
Local oversight
- 2.1.10 The audit team also observed instances where there was either no local committee in place, local committees did not meet as required, or joint local committee (with other research centres) were not providing program oversight as required to ensure that the risks of using the Level 2 pathogens are being adequately mitigated.
Laboratories using Human and Animal Pathogens (Containment Level 2 (CL-2))
- 2.1.11 Regulators for Containment Level 2 (CL-2) laboratories (Public Health Agency of Canada (PHAC)) require that a checklist be completed every two years and submitted by the BSO and signed-off by research centre management in order to obtain approval for the use of Level 2 pathogens. This checklist includes the requirement to inform the regulator as to whether or not project risk assessments are completed at the research centre.
- 2.1.12 Risk assessments must be completed prior to the commencement of a science project that plans to use a Level 2 pathogen, and outline (among other things) the possible risks of using a Level 2 pathogen. Standard Operating Procedures (SOPs) are developed to identify procedures and processes that can be implemented to mitigate the risks identified in the risk assessment. Both the risk assessment and all accompanying project related SOPs must be reviewed and approved by the local CBB committee prior to project implementation.
- 2.1.13 Internal Audit observed instances where project risk assessments for the CL-2 laboratories were not completed despite having notified the regulatory agency that risk assessments were completed. Following the conduct phase of the audit, the audit team was advised that steps had been taken to complete the risk assessments at the identified laboratories.
Laboratories using Plant Pest Pathogens (PPC-2)
- 2.1.14 At the time of the audit, the regulators for Plant Pathogen Containment Level 2 (PPC-2) laboratories (Canadian Food Inspection Agency (CFIA)) did not require the completion of project risk assessments, however the requirement is found in the AAFC guidelines. The audit team noted that risk assessments had not been completed by the PPC-2 laboratories visited during the conduct of the audit.
-
2.1.15 Recommendation 1: Assistant Deputy Minister (ADM), Science and Technology Branch (STB) should implement timely and appropriate mechanisms to ensure compliance with both regulatory requirements and AAFC guidelines, including conducting and completing risk assessments and to assess the state of program implementation across STB on an ongoing basis.
Management response: agree
Action plan:
Science and Technology Branch (STB) will revise the program documentation to clarify roles and responsibilities of all individuals involved in the CBB program and the authority of governance bodies.Science and Technology Branch (STB) will review the existing data collection processes, including the risk assessments, to ensure data received from Centres is complete, implement a reporting schedule on an ongoing basis and ensure timely review of the information received in order to determine compliance with regulations and guidelines.
Science and Technology Branch (STB) will also dedicate a resource to ensure that the documentation is performed and completed, as well as identify an STB DG level champion for the CBB program.
Lead(s) responsible: ADM, STB
Target date for completion: September 30, 2015
-
2.1.16 Recommendation 2: Assistant Deputy Minister (ADM), STB should ensure that formal periodic reporting be made to branch management, and to the AAFC National Occupational Health and Safety Policy Committee (NOHSPC), on the status of the CBB program implementation, the achievement of CBB policy objectives, the number and location of Level 2 laboratories in the department and other relevant changes/developments that have occurred.
Management response: agree
Action plan:
The NCBBC will provide a formal report on the status of the CBB Program, the achievement of CBB policy objectives, the number and location of level 2 laboratories in the department and other relevant changes/developments that have occurred to the STB Branch Executive Committee (BEC) annually. Emerging issues will be reported to the ADM of STB within 48 hours. To improve communication and awareness of the Containment, CBB program, reports will be shared with the NOHSPC.Lead(s) responsible: ADM, STB
Target date for completion: October 31, 2015
2.2 Roles and responsibilities
- 2.2.1 The audit expected that roles and responsibilities for employees with responsibilities under the Agriculture and Agri-Food Canada's (AAFC) Containment, Biosafety and Biosecurity (CBB) Program were clearly defined, communicated and implemented.
- 2.2.2 Review of documentation confirmed that roles and responsibilities are communicated through the guidelines, manuals, Standard Operating Procedures (SOPs), supervisors and/or training.
- 2.2.3 However, roles and responsibilities as defined in the CBB guidelines and CBB policy are not always clear and could benefit from clarification as it relates to the responsibility to conduct program self-assessments. As well, the committee Terms of Reference (TOR) and program policy have not been updated since 2009 to ensure compliance with the revised guidelines and to ensure clarity of roles and responsibilities.
- 2.2.4 The audit team noted that the majority of roles and responsibilities were implemented; however, in some instances, there was a lack of implementation and in some cases non-compliance with both regulatory and AAFC guidelines were noted.
- 2.2.5 As noted above, the audit found that project risk assessments had not been conducted at three of the Containment Level 2 (CL-2) laboratories and two Plant Pathogen Containment (PPC) laboratories. The three CL-2 laboratories have since implemented the use of risk assessments.
- 2.2.6 The Public Health Agency of Canada (PHAC) guidelines define the record keeping requirements for Pathogen Accountability (inventory of Level 2 pathogen samples). Inconsistencies were observed at the four sites visited in terms of the level of detail documented to track pathogens and in some cases did not fully comply with guidelines. The audit found there is a lack of awareness of what is required to meet the regulatory requirements and/or AAFC's guidelines to ensure the inventory list of pathogens is maintained current.
-
2.2.7 Recommendation 3: Assistant Deputy Minister (ADM), Science and Technology Branch (STB) should ensure that roles and responsibilities are reviewed in conjunction with the revision of the AAFC CBB policy to ensure they are consistent with other CBB documentation (such as the CBB guidelines and the National Containment, Biosafety and Biosecurity Committee (NCBBC) TOR) and that a formal communication (to technicians, scientists, Biosafety and Biocontainment Officers (BSO/BCO) and Associate Director, Research, Development and Technology (AD, RDT)) is developed and implemented to improve awareness to regulatory requirements and AAFC guidelines.
Management response: agree
Action plan:
The AAFC CBB Policy will be updated to reflect the current organizational structure and the roles and responsibilities to senior management positions.Science and Technology Branch will review, update and revise the program documentation to clarify roles and responsibilities of individuals involved in the CBB program and the authority of governance bodies to ensure consistency. Formal communications will be developed and implemented to ensure compliance and improve awareness after the program documentation has been updated.
Lead(s) responsible: Assistant Deputy Minister (ADM), Science and Technology Branch (STB)
Target date for completion: October 30, 2015
2.3 Training
- 2.3.1 The Agriculture and Agri-Food Canada (AAFC) guidelines recommend that an annual Containment, Biosafety and Biosecurity (CBB) safety seminar be provided to employees working directly with pathogens and requires two types of training under the AAFC CBB program:
- Basic National training (for example Public Health Agency of Canada (PHAC) training modules);
- Research Centre and project specific training (for example local procedures manuals, Standard Operating Procedures (SOP)).
- 2.3.2 The audit team expected to find that all individuals working in a Level 2 laboratory including employees and non-employees (for example students and visiting scientists) are trained in accordance with regulations and guidelines and that basic CBB training is provided prior to working independently in a Level 2 laboratory. Furthermore, Internal Audit expected to find that training is monitored, tracked and updated on a regular basis.
- 2.3.3 The audit team determined that for the Level 2 laboratories visited during the audit, the majority of employees received basic training prior to commencing work in the Level 2 laboratories.
- 2.3.4 However, a few instances of a lack of compliance with either regulatory and/or AAFC guidelines were noted at 2 of the 4 centres visited by the audit team, in the following areas;
- Processes were not in place to ensure that employees and non-employees received training for project specific SOP, the Local procedural manual, and pathogen Material Safety Data Sheets (MDSDs) and therefore were not receiving all of the required training;
- Employee attestation was not obtained to ensure that the training material was understood; and,
- Internal Audit observed that the annual CBB safety seminars that are recommended by the AAFC CBB guidelines were not provided to employees.
- 2.3.5 As per the AAFC guidelines, training material is required to be updated every 3 years (or more frequently as determined by the nature of the work being performed) and SOP should be reviewed and updated (if required) on an annual basis by the author. Training material at the national level and at the majority of research centres, visited during the audit, is updated on a regular basis. However, the audit team did not observe evidence that the SOP were consistently reviewed on an annual basis.
- 2.3.6 The audit team would have expected to find that employees working in Level 2 laboratories updated their CBB training on a regular basis that is consistently implemented across the CBB program. The AAFC CBB guidelines state that training plans should be reviewed at least annually or whenever new activities are assigned but do not specify the frequency with which employees should update their training. The audit team observed that no consistent process is in place to ensure that employees (and long term non-employees) update their training on a regular basis.
- 2.3.7 At the time of the audit report, steps have been taken by some research centres to update training processes in an effort to become compliant with the guidelines.
-
2.3.8 Recommendation 4: Assistant Deputy Minister (ADM), Science and Technology Branch (STB) should ensure that the training and re-training requirements under the AAFC (CCB) Program are reviewed, updated and communicated to ensure a consistent approach is adopted across STB and that the training program is delivered to employees and non-employees working in Level 2 laboratories and monitored on a regular basis.
Management response: agree
Action plan:
The National Containment, Biosafety and Biosecurity Committee (NCBBC) will review, update, and communicate training requirements to Research Centres. Training and re-training activities will be reported to the NCBBC by Research Centres on an annual basis and monitored to ensure the program is delivered consistently to employees and non-employees working in level 2 laboratories.Lead(s) responsible: ADM, STB
Target date for completion: November 30, 2015
2.4 Lessons learned
- 2.4.1 The audit team expected to find that processes have been established within the Containment, Biosafety and Biosecurity (CBB) program to share best practices and lessons learned.
- 2.4.2 Internal Audit observed evidence that mechanisms have been established at the national level to share best practices and/or issues by way of the national committee meetings and the regular open call meetings with Biosafety and Biocontainment (BSO / BCO) officers.
- 2.4.3 The audit team did observe areas where the sharing of information can be improved in an effort to help to inform research centres of their responsibilities for the implementation of program requirements and avoid potential duplication of program non-compliance, accidents and/or near misses across the program.
- 2.4.4 The results of program self-assessments are shared with the national committee Chair and with those individuals who submitted the self-assessments at the research centre. Neither the national committee members nor other research centres are advised on the results/findings of the self-assessments.
- 2.4.5 In the event of an accident or 'near miss' in a Level 2 laboratory a "Near Miss or Incident/Accident Report" is prepared and submitted to (including but not limited to) the National Containment, Biosafety and Biosecurity Committee (NCBBC). No reports had been prepared at the locations visited or submitted to the national committee during the period under review or during the conduct of the audit. However, the audit team did receive and review reports submitted to the NCBBC following the conduct phase of the audit.
- 2.4.6 The completed reports include action plans/recommendations proposed by the research centre help avoid the incident from re-occurring. Given the importance of the information included in the reports, it is the opinion of the audit team that the action plans/recommendations should be shared across the program, on an ongoing basis, in an effort to help mitigate similar situations occurring at other AAFC locations. At the time of the conduct phase of the audit, the information was not shared across the program. The NCBBC has since included the above mentioned 'near miss' as a regular agenda item at its meetings.
-
2.4.7 Recommendation 5: Assistant Deputy Minister (ADM), Science and Technology Branch (STB) should enhance existing mechanisms for the sharing of best practices/lessons learned to ensure that findings from program self-assessments and "near miss" and/or incident/accidents are communicated across the AAFC CBB Program, on an ongoing basis.
Management response: agree
Action plan:
The NCBBC recently revised the format of existing open-line teleconferences by recording meeting minutes in addition to an open-line segment, the agenda includes a number of standing items such as the sharing of best practices/lessons learned from program self-assessments and "near miss" and/or incident/accidents to facilitate information sharing.Science and Technology Branch (STB) will also complete the development of its Knowledge Workspace (KW) site to ensure ease of access to shared documents for all individuals involved.
Lead(s) responsible: Assistant Deputy Minister (ADM), Science and Technology Branch (STB)
Target date for completion: November 30, 2015
Annex A: Audit criteria
Governance:
- 1.1 Management is provided with appropriate information (sufficiency, completeness, timely and accurate).
- 1.2 Roles and responsibilities are clearly defined, communicated and implemented.
- 1.3 Processes have been established to share best practices and lessons learned.
- 1.4 Agriculture and Agri-Food Canada (AAFC) laboratories are managed, maintained and monitored at the appropriate level (as per guidelines) to ensure compliance, efficiency and effectiveness.
- 1.5 Succession planning and knowledge transfer process for the program has been established.
Safeguarding of Assets:
- 2.1 A process is in place to track and or reconcile (on a regular basis) assets within laboratories (autoclaves, microscopes, hood fans, etc.) to ensure AAFC assets are safeguarded.
- 2.2 Pathogen accountability (sample inventory control) of research samples at research centres is being recorded and monitored (kept up to date) on a regular basis.
- 2.3 Procedures in place are adequate and appropriate to ensure that access to Level 2 laboratories (and the contents within the laboratory) is limited to authorized individuals.
Training:
- 3.1 Employees and non-employees are trained in accordance with regulations and guidelines as it relates to biosafety, biosecurity and safe guarding of assets, et cetera.
- 3.2 Training is monitored, tracked and updated on a regular basis to ensure that AAFC laboratory employees have up-to-date training.
Compliance:
- 4.1 Agriculture and Agri-Food Canada (AAFC) Level 2 laboratories are in compliance with the AAFC Containment, Biosafety and Biosecurity (CBB) guidelines and other applicable regulatory guidelines (such as Public Health Agency of Canada (PHAC) & Canadian Food Inspection Agency (CFIA)) in areas such as:
- Ensuring that processes are in place to monitor the disposal of infectious materials.
- Ensuring that approval is obtained from regulatory bodies prior to importing or transferring pathogens.
- Ensuring that approval is obtained from regulatory bodies for the use of Level 2 pathogens.
- Ensuring that all incidents, accidents or "near misses" within Level 2 laboratories are investigated and reported to management.
Annex B: Acronyms
- AAFC
- Agriculture and Agri-Food Canada
- ADM
- Assistant Deputy Minister
- AD, RDT
- Associate Director, Research, Development and Technology
- BCO
- Biocontainment Officer
- BSO
- Biosafety Officer
- CBB
- Containment, Biosafety and Biosecurity
- CFIA
- Canadian Food Inspection Agency
- CL-2
- Containment Level 2 (Animal and Human Pathogens)
- CMB
- Corporate Management Branch
- HPTA
- Human Pathogen Toxin Act
- IA
- Internal Audit
- IIA
- Institute of Internal Auditors
- LCBBC
- Local Containment, Biosafety and Biosecurity Committee
- MSDS
- Material Safety Data Sheets
- NCBBC
- National Containment, Biosafety and Biosecurity Committee
- NOHSPC
- National Occupational Health and Safety Policy Committee
- PHAC
- Public Health Agency of Canada
- PPA
- Plant Pathogen Act
- PPC-2
- Plant Pathogen Containment Level 2
- OAE
- Office of Audit and Evaluation
- SOP
- Standard Operating Procedure
- STB
- Science and Technology Branch
- TOR
- Terms of Reference
Annex C: Reporting structure
Source: Prepared by Internal Audit
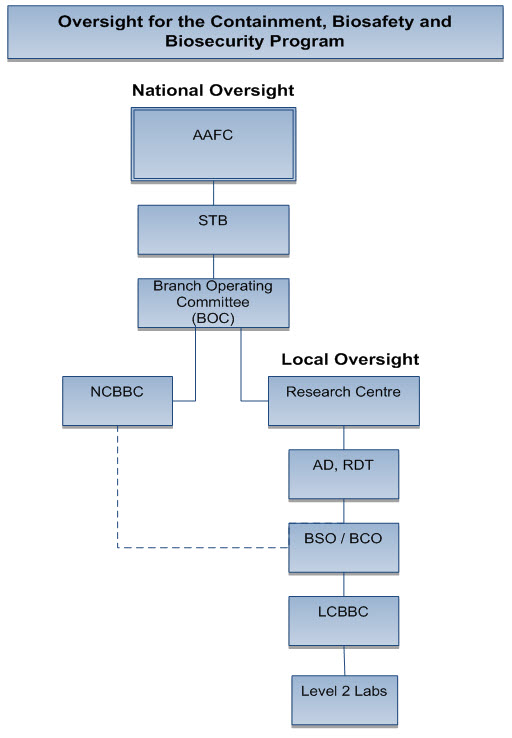
Description of above image
The table illustrates the reporting structure for oversight for the containment, biosafety and biosecurity program. The contents of the table are as follows:
- National oversight:
Agriculture and Agri-Food Canada (AAFC) oversees Science and Technology Branch (STB); STB oversees the Branch Operating Committee (BOC) which oversees the National Containment, Biosafety and Biosecurity Committee (NCBBC) and Research Centers; NCBBC unofficially oversees Biosafety Officer / Biocontainment Officer (BSO / BCO) within Research Centers; - Local oversight at the Research Centre:
Associate Director, Research, Development and Technology (AD, RDT); oversees BSO / BCO who oversees the Local Containment, Biosafety and Biosecurity Committee (LCBBC); LCBBC oversees Level 2 Labs.
Annex D: Human and animal pathogens
Source: Canadian Biosafety Standards and Guidelines
Human and Animal pathogens are assessed and categorized into risk groups by Public Health Agency of Canada (PHAC) using criteria from Human Pathogen Toxin Act (HPTA) and takes into consideration the level of risk to the health of a person or to public health, as well as the likelihood that the human pathogen will cause disease in a human, and whether or not treatment and preventative measures are available.
Pathogens used in Containment Level 2 (CL2) laboratories having the following definition as per Public Health Agency of Canada (PHAC):
Risk Group 2 (moderate individual risk, low community risk)- Any pathogen that can cause human disease but, under normal circumstances, is unlikely to be a serious hazard to laboratory workers, the community, livestock or the environment. Laboratory exposures rarely cause infection leading to serious disease; effective treatment and preventive measures are available, and the risk of spread is limited.
An example of a pathogen that would fall under risk Level 2 for a containment lab would be salmonella.
Containment Level 2 (CL-2)
Biosafety and biosecurity at CL2 laboratories are achieved through operational practices and physical containment that are proportional to the risks associated with the pathogens being handled. CL2 builds upon the basic laboratory practices that are established for level one laboratories.
Operational practices for CL2 include but are not limited to:
- administrative controls (for example, biosafety program management, training) and
- procedures (for example, work practices, Personal Protective Equipment use, decontamination) that mitigate the risks associated with the activities conducted within the zone.
Physical containment features include but are not limited to:
- facility design (for example, location, surface finishes, access control) and
- biosafety equipment, such as primary containment devices (for example, BSCs) for certain activities.
Annex E: Plant pest pathogens
Source: Canadian Food Inspection Agency (CFIA) Containment Standards for Facilities Handling Plant Pests and Canadian Biosafety Standards and Guidelines
Plant pests almost never infect or infest healthy people, and they therefore pose little direct risk to laboratory personnel. Some can, however, pose a threat to agricultural production, forests and natural environments. As a result, it is important that personnel working with plant pests and the facilities housing these organisms take steps to prevent the accidental escape of potentially damaging pests into the environment.
Plant Pathogen Containment Level 2 (PPC-2)
Plant Pathogen Containment Level 2 (PPC-2) facilities include permanent structures such as laboratories and greenhouses but not screenhouses. Containment is achieved through facility design, operational procedures and the use of specialized equipment. All PPC-1 physical and operational requirements also apply to this containment level.
Operational practices for Plant Pathogen Containment Level 2 (PPC-2) laboratories include but are not limited to:
- use of primary containment devices;
- use of dedicated or disposable laboratory clothing;
- appropriate decontamination of solid and liquid waste;
- pest monitoring and regular inspection of screens, filters and caulking for defects;
- clear documentation of standard operating procedures (SOPs);
- mandatory personnel training; and
- the availability of suitable emergency response plans.
Physical requirements for Plant Pathogen Containment Level 2 (PPC-2) laboratories include but are not limited to:
- restricted access via an anteroom;
- an on-site autoclave; and
- greenhouses that are mechanically ventilated with screened or filtered inlet and exhaust air.