1. Purpose
- 1.1 As genetically modified (GM) grain is authorized for commercial use and cultivated, harvested, transported and stored, trace amounts of that grain may become mixed with other grain varieties, despite the use of best management practices by industry. As a result, a GM grain that has not yet been approved by the importing country may unintentionally be present, at low levels, in grain shipments exported to that country. This is what is called low level presence (LLP).
- 1.2 The LLP policy model has been designed to stimulate domestic and international discussions on the management of unauthorized GM crops in imported grain, food and feed, using a risk-based enforcement approach that would minimize LLP-related trade disruptions.
- 1.3 The LLP policy model includes the combination of: 1) proactive actions to mitigate the potential risk posed by LLP, and; 2) a risk-based enforcement approach to provide enhanced transparency and predictability on how LLP situations would be addressed.
2. Definitions
- 2.1 For the purpose of this LLP policy model:
- Genetically modified (GM) refers to organisms that have been modified using recombinant Deoxyribonucleic Acid (DNA) technology.
- GM crop refers to a plant with one or more traits that have been introduced via recombinant DNA technology.
- Low level presence (LLP) is the unintentional presence, at low levels, of unauthorized GM crops in imported grain, food or feed; where the GM crop is authorized for food use in one or more foreign jurisdictions but is not authorized in the importing country.
3. Objectives
- 3.1 The LLP policy model features policy elements designed to :
- minimize disruptions to trade while protecting the health and safety of humans, animals and the environment;
- provide policymakers and regulators with tools to develop a pragmatic and efficient risk-based approach to managing LLP; and,
- enhance transparency and predictability for importers and exporters on how LLP situations will be managed.
4. Guiding principles
- 4.1 The following principles have supported the development of the LLP policy model. They may also guide the development of risk-based enforcement approaches to managing LLP situations:
- The safety of human food, animal feed and the environment is paramount.
- LLP policy model requirements encourage compliance with domestic regulations, in particular by requiring technology developers to seek authorizations for new GM products.
- Risk management decisions and enforcement actions are risk-based and minimize unnecessary trade disruptions.
5. Scope
- 5.1 To be trade-facilitative, LLP solutions should ideally apply to imported whole grain for food and feed use, as well as imported food and feed products derived from grains.
- 5.2 This LLP policy model is not intended to apply to:
- seed intended for propagation in the environment;
- GM fruits and vegetables;
- adventitious presence which is defined, for the purpose of this Policy, as the unintended release of GM crops that have not been authorized for use in any foreign jurisdiction;
- GM animals and microorganisms;
- other GM crops modified to produce plant-made pharmaceutical or industrial products unless approved for food and feed use.
- 5.3 LLP policy solutions should not be implemented in a way that supersedes any varietal purity, organic or other such agricultural standards.
6. Eligibility criteria
- 6.1 To be considered LLP, an unauthorized GM crop must meet two criteria. These eligibility criteria have been established to proactively mitigate potential risks posed by low levels of unauthorized GM crops:
- i. The GM crop must be approved for food use in a least one country, in accordance with the Codex Guideline for the Conduct of Food Safety Assessment of Foods Derived from Recombinant-DNA Plants (CAC/GL 45-2003).
- ii. Appropriate test methodologies and reference materials for the detection and identification of the specific GM crop should be made available to the importing country to support monitoring of LLP in imports.
7. Risk management approach for LLP in grain for food and feed use
- 7.1 A GM crop that meets the eligibility criteria of the policy model is considered LLP, and should be managed using a graduated risk-based approach based on two levels (see figure in Appendix 1).
- The graduated risk-based approach applies to all crop types.
- 7.2 A level of 0.2% is established to manage LLP at concentrations below or equal to 0.2% and is designed to address situations such as LLP resulting from dust, lingering traces of discontinued varieties, or foreign crops intended for domestic use only.
- The enforcement response to an LLP situation at or below 0.2% should reflect the negligible risk posed by the non-compliance.
- The enforcement response could include compliance promotion actions including, for example, advising the regulated party, in writing, of the LLP detection.
- 7.3 A compliance threshold of 3% is established to manage LLP at concentrations between 0.2% and 3% and is designed to address LLP situations resulting from the commercialization of a GM crop in situations of asynchronous approvals.
- The following two additional criteria should be met for the compliance threshold to apply:
- i. Application for the authorization of the GM crop should have been submitted to the importing country.
- The proactive submission of an application is intended to limit occurrences of asynchronous approvals as well as their duration.
- It confers a temporary status to the compliance threshold, as return to compliance will be ultimately achieved through the authorization of the GM crop.
- ii. Applicable LLP risk assessment(s) should have determined, in advance, that the GM event does not pose a risk, should it be present in an import shipment at levels below the compliance threshold.
- i. Application for the authorization of the GM crop should have been submitted to the importing country.
- The enforcement response to an LLP situation between 0.2% and 3% will reflect the minimal risk associated with the non-compliance, as determined by the LLP risk assessments.
- The enforcement response could include appropriate actions such as issuing a letter of non-compliance to the regulated party.
- The following two additional criteria should be met for the compliance threshold to apply:
- 7.4 When the unauthorized GM crop does not meet all of the policy and compliance threshold eligibility criteria, the LLP policy model would not apply.
- In these situations, the importing country should determine the appropriate control and/or enforcement response.
8. Roles and responsibilities
The implementation of a comprehensive LLP policy would require efforts from government entities as well as various players of the grain supply chain.
- 8.1 Importers of grain, food and feed products would be responsible for:
- ensuring that products imported into the country comply with relevant regulatory requirements.
- 8.2 GM technology developers would be responsible for:
- seeking authorization for the GM crop by submitting an application; and,
- providing detection methods and reference materials to regulators of the importing country.
- 8.3 Regulatory agencies of the importing country would be responsible for:
- administering their mandated legislation and implementing the LLP policy model.
9. Monitoring activities
- 9.1 Frequency of monitoring of imported grain should be consistent with the negligible risk posed by LLP crops.
- 9.2 Monitoring samples of grain shipments may be taken and analyzed for the presence of LLP. The standard principles of measurement uncertainty resulting from laboratory testing variation would be applied to the raw test results when they are interpreted to determine the detected LLP levels.
10. References
- 10.1 Codex Alimentarius Commission CAC/GL 45-2003 Guideline For The Conduct Of Food Safety Assessment Of Foods Produced Using Recombinant-DNA Plants (external PDF) (including Annex 3: Food Safety Assessment in Situations of Low level Presence of Recombinant-DNA Plant Material in Food)
Appendix 1
Graduated risk-based approach to managing LLP
The following figure illustrates the eligibility criteria that must be met by a GM crop to be considered LLP in order to be managed using the graduated risk-based approach featured in this policy model.
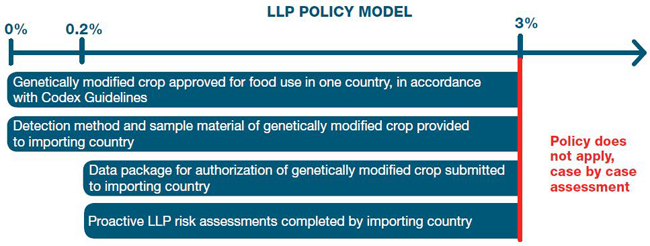
Description of image above
LLP Policy Model
To be considered low level presence under the policy model, concentrations of a genetically modified crop between 0% and 3% must meet two criteria:
- Genetically modified crop approved for food use in one country, in accordance with Codex Guidelines
- Detection method and sample material of genetically modified crop provided to importing country
Concentrations of a genetically modified crop between 0.2% and 3% must meet two additional criteria:
- Data package for authorization of genetically modified crop submitted to importing country
- Proactive LLP risk assessments completed by importing country
Over 3%:
- Policy does not apply, case by case assessment