Authors
Odile Carisse, Ph.D., Phytopathology, and Tristan Jobin, M.Sc.
Agriculture and Agri-Food Canada
Horticulture Research and Development Centre
With the collaboration of
- Wendy McFadden-Smith, Ph.D.
McSmith Agricultural Research Services - Catherine Meloche, M.Sc.
Agriculture and Agri-Food Canada - Jacques Lasnier
Co-Lab Research and Development
Introduction
In eastern Canada, apple scab is of major economic importance. It occurs every year and often causes significant economic losses. Losses result directly from fruit infections which affect yield, fruit quality and storability or indirectly from defoliation which can affect tree vigour and winter survival.
Apple scab management is most often based on repeated fungicide applications that result in high costs in terms of money for the fungicide sprays and in time dedicated to scab management. Because of increasing pressure on apple growers to reduce pesticide use and reduce production costs, while maintaining a high level of crop quality, we believe it is crucial and timely to simplify and optimize apple scab management.
Moreover, development of resistance to some fungicides may complicate the situation further. Apple scab management is based on fungicide applications. However, some fungicides could lose their efficacy following the development of resistance in the fungus causing apple scab. The loss of these fungicides, mainly used as post-infection sprays, may have major consequences, among which is increased usage of pre-infection fungicides that are more harmful to the environment.
The objectives of this publication are:
- (1) to summarize the current knowledge on apple scab,
- (2) to present new tools for scab management, and
- (3) to simplify the use of these tools in an integrated management program.
Components of apple scab management
- Primary inoculum management
- Forecasting risk: primary infections
- Host growth and susceptibility
- Fungicide resistance
- Production system
- Forecasting risk: primary inoculum
- Fungicide sprays
- Sanitation
Biology: symptoms
Apple scab is caused by the fungus Venturia inaequalis. This disease also affects crabapple. Development of apple scab is favoured by rainy, humid and cool spring weather conditions. If it is not properly controlled, it will cause scab lesions on fruits that will reduce fruit quantity and value.
Symptoms
Leaves and fruits are highly susceptible to apple scab when they are young and growing. Mature leaves and fruits gain resistance as they age. During the main growth period in early spring, there is more susceptible tissue available for infection and therefore, greater risk of disease than later in the season.
Leaves
Young scab lesions are pale, irregular, and small. As they age, they become circular and olive-colored with a velvety texture. Lesions in a more advanced stage become black and slightly raised. Heavily scabbed leaves can desiccate, become deformed and fall.
Flowers
Lesions on flowers resemble those on the leaves. A single lesion on the pedicel or a sepal can make the flower desiccate and fall.
Fruits
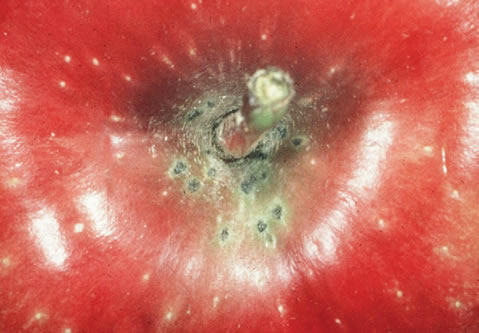
Early infections can lead to abnormal growth (fruit deformation) and fruit drop. The lesions on the fruits are similar to those on the leaves, but as they age, they will produce cracks. If the fruit is infected late in the summer or just before harvest, black, circular, very small (0.1 - 4 mm diameter) lesions called 'pin-point scab' will appear during storage.
Apple scab generally does not kill the trees but it can cause defoliation, which will weaken the tree and influence its survival during winter conditions.
Apple scab lesion in the early stage of development

Secondary scab lesions on leaves


Scab lesions on fruits


Pin-Point Scab
Biology: Pathogen life cycle
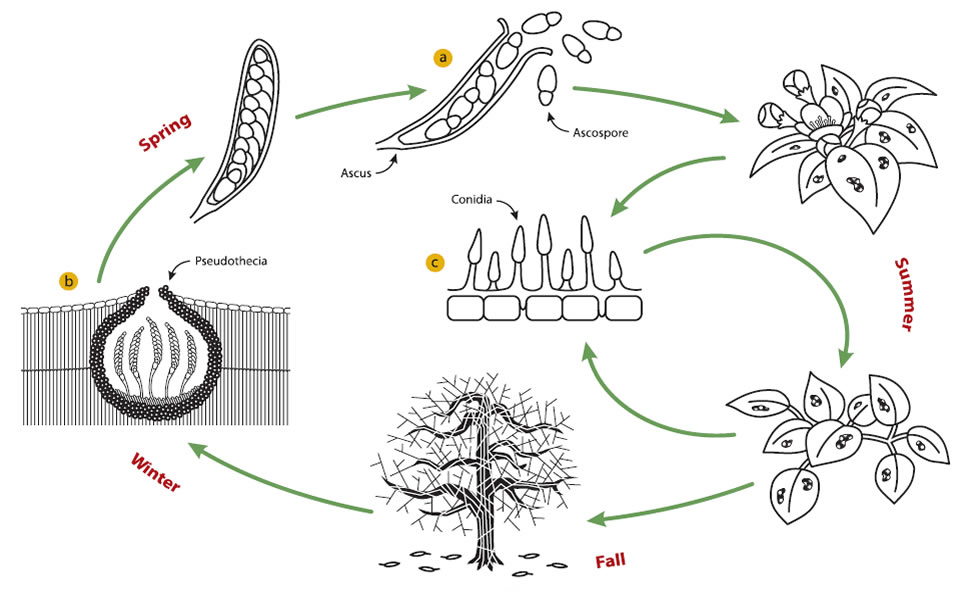
Venturia inaequalis is a microscopic fungus from the ascomycetes family, meaning that it produces reproductive spores in a bag called an ascus (a).
In autumn, after leaf fall, the fungus colonizes the leaves and sexually produces survival structures called pseudothecia that will protect it against winter conditions (b).
In the spring, when temperature increases, the fungus produces small spores called ascospores in the little bags (asci) that develop within the pseudothecia. When mature, the ascospores are ejected into the air during a rain or heavy dew event and dispersed by wind and rain.
When ascospores are deposited on a young and susceptible leaf or fruit and weather conditions are favourable, they rapidly germinate and produce mycelium to form brownish lesions.
This period of primary infections, i.e. the period when ascospores are produced, usually lasts six to eight weeks, starting approximately at green tip stage and ending around the middle or end of June, about two weeks after fruit-set.
Later, the lesions take a velvety appearance, indicating that the mature mycelium is producing conidia (c), sometimes called summer spores. These spores are dispersed mainly by rain and wind. When these spores are deposited on wet leaves or fruits, they cause new lesions that produce new conidia. These secondary infections can occur all summer long if the weather conditions are favourable.
The preventive approach
Better safe than sorry:
Cultivar
For new orchards, choose tolerant or moderately susceptible cultivars (see Susceptibility of Apple Cultivars below).
Pruning
Good pruning allows good tree aeration, which causes leaves to dry more quickly. It also allows for better fungicide coverage.
Fertilization
A good fertilization program is a must, but an excess of nitrogen-based fertilizers may result in the growth of sucker shoots that are highly susceptible to apple scab. Irrigation during dry periods will help maintain tree vigor.
Inoculum reduction
The fungus overwinters in dead leaves on the orchard floor, so destruction of the leaf litter by burning, mowing or soil tilling contributes to the reduction of primary inoculum. An application of urea or fungal antagonists also reduces the winter survival of V. inaequalis and hence, reduces scab-risk the following season.
Susceptibility of apple cultivars
- Highly susceptible
- 'McIntosh';
- 'Vista Bella'
- Susceptible
- 'Cortland';
- 'Red Cortland' (RedCort);
- 'JerseyMac';
- 'Jonamac';
- 'Lobo'
- Moderately susceptible
- 'Early Geneva';
- 'Empire';
- 'Golden Delicious';
- 'Jonagold';
- 'Lodi';
- 'Mutsu';
- 'Northern Spy';
- 'Spartan';
- 'Royal Gala'
- Low susceptibility
- 'Golden Russet';
- 'Idared';
- 'Red Delicious';
- 'Sunrise';
- 'Paulared'
- Resistant
- 'Belmac',
- 'Enterprise',
- 'Freedom',
- 'Florina' (Querina),
- 'Jonafree',
- 'Liberty',
- 'Macfree',
- 'Nova Easygro',
- 'Novamac',
- 'Primevere',
- 'Pristine',
- 'Redfree',
- 'Richelieu',
- 'Rouville',
- 'Supermac'
Autumn
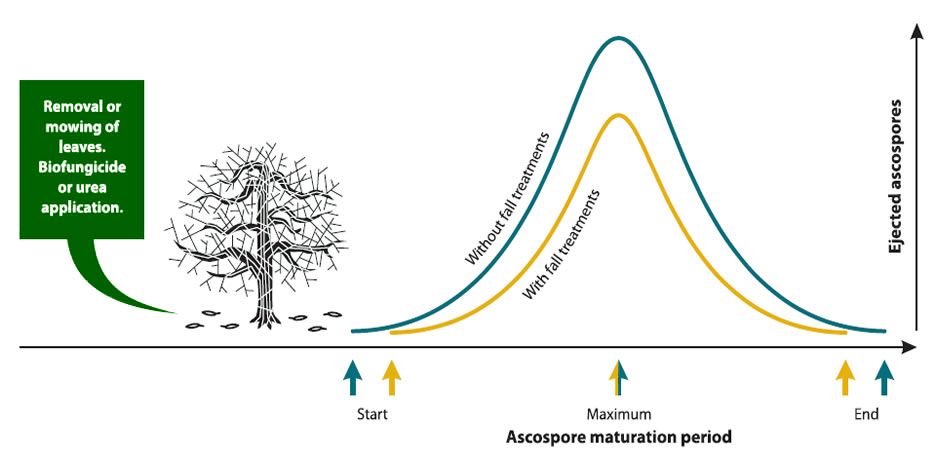
Reduction of primary inoculum: Fall treatments
Fall treatments include leaf shredding, mulching, burning or removal of the leaf litter and the application of urea or a biological control agent.
Leaf shredding/mulching involves blowing the leaves from row "middles" into the row alleys and then shredding with a flail mower. This mechanical sanitation and the application of urea or biological control agents to the leaf litter can be performed when most of the leaves have fallen and again in early-spring, about three to four weeks before bud break for maximum effectiveness. Depending on the time of leaf fall and the beginning of snow cover, it may not be possible to do these treatments every year.
These treatments allow a reduction in the quantity of ascospores that will be produced in the spring, and research has shown that these sanitation procedures can reduce ascospores as much as 95%.
In orchards planted with susceptible cultivars and in severely scabbed orchards, the number of fungicide applications needed to control scab in the spring should not be reduced. However, if fall treatments are performed, scab infection-risk on leaves and fruits will be much lower, and hence facilitate management of primary scab.
In orchards with low levels of apple scab and/or planted with less-susceptible cultivars, fall treatments may allow a reduction in fungicide sprays in the spring.
Regardless of the type of fall treatment, the ascospore maturation pattern will not change, but the quantity of ascospores produced will be lower (see graph below). Consequently, the ascospore maturation model can still be used.
Estimation of the potential amount of primary inoculum
There is a method named potential ascospore dose or 'PAD', that estimates the quantity of ascospores based on a sampling of the leaves in late autumn, but before the leaves fall. The estimation predicts the next spring overall "scab-risk" as high or low.
Sequential sampling plan for autumn scab
When: after harvest but before leaf fall
If you use the MacHardy method I: for large trees, count the number of scabbed leaves on 10 trees, 10 shoots per tree. For small trees, count the number of scabbed leaves on 20 trees, 5 shoots per tree. If you use the simplified method II, select 100 shoots.
- Randomly select the shoots within the orchard block, select both clusters and terminals shoots, all from parts of the tree canopy (high, low, exterior, and interior). If sucker shoots are present, make sure to select some sucker shoots.
- If the number of scabbed leaves is above the threshold (red zone), a full fungicide program will be needed the following spring. All primary infections should be controlled.
- If the number of scabbed leaves is above the threshold (red zone), a full fungicide program will be needed the following spring. All primary infections should be controlled.
- If the number of scabbed leaves is in the intermediate zone (orange zone), sampling must continue with another series of shoots before a decision can be made. Sample another series of 10 or 20 trees if you use the method proposed by MacHardy or another series of 10 shoots if you use the simplified method.
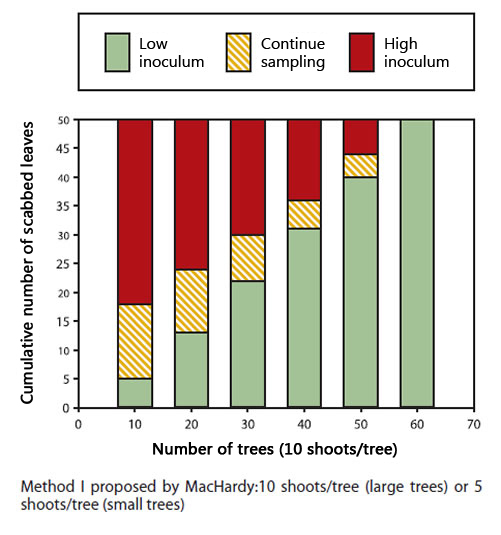
Description of above image
Method I proposed by MacHardy: 10 shoots/tree (large trees) or 5 shots/tree (small trees). The following table presents the cumulative number of scabbed leaves by the number of trees (10 shoots/tree) and identifies the corresponding zone.
| Number of trees (10 shoots/tree) | Low inoculum (green zone) | Continue sampling (orange zone) | High inoculum (red zone) |
|---|---|---|---|
| 10 | 0-5 | 6-18 | 19-50 |
| 20 | 0-13 | 14-23 | 24-50 |
| 30 | 0-22 | 23-30 | 31-50 |
| 40 | 0-31 | 32-36 | 37-50 |
| 50 | 0-40 | 41-44 | 45-50 |
| 60 | 0-50 |
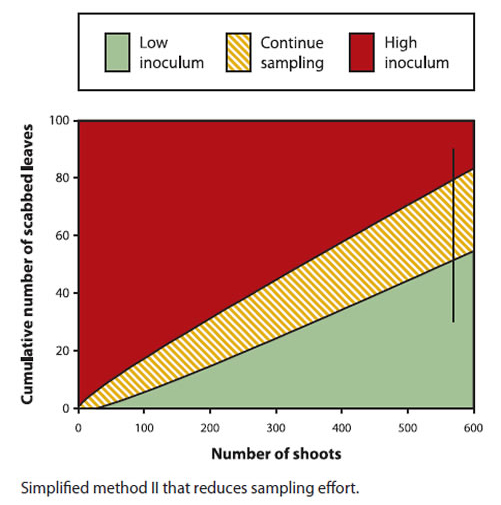
Description of above image
Simplified method II that reduces sampling effort. The following table presents the cumulative number of scabbed leaves by the number of shoots and identifies the corresponding zone.
| Number of shoots | Low inoculum (green zone) | Continue sampling (orange zone) | High inoculum (red zone) |
|---|---|---|---|
| 100 | 0-6 | 7-18 | 18-100 |
| 200 | 0-14 | 15-32 | 33-100 |
| 300 | 0-22 | 23-43 | 44-100 |
| 400 | 0-35 | 36-58 | 59-100 |
| 500 | 0-43 | 44-70 | 71-100 |
| 600 | 0-55 | 56-81 | 82-100 |
Spring
Primary infections: Estimation of infection risks
Apple scab is a disease that develops in two phases: primary infections caused by ascospores and secondary infections caused by conidia. Young leaves are susceptible from their emergence to the age of about 8 days, after which they will gradually become less susceptible.
Primary infections
Primary infections are caused by ascospores, with the quantity of ascospores produced directly related to the level of scab the previous fall and weather conditions during the winter.
Thus, the higher the scab level in the orchard in the previous fall, the higher the risk of primary infections the following spring.
This means that any sanitation practice or treatment that will reduce the overwintering stage of the fungus will directly reduce the risk of scab in the spring.
Also, since the number of ascospores is limited and no new ascospores are formed in the spring, it is very important to efficiently control primary infections to avoid secondary infections later in the season.
How to identify the severity of an apple scab infection
Infection severity or the number of scab lesions that develop depends on the quantity of ascospores, leaf or fruit susceptibility, and weather conditions, mainly temperature and leaf wetness duration. Infection risk is calculated based on those two weather criteria (see table below).
The duration of leaf wetness is calculated in hours starting at the beginning of a rain when the leaves are wet and ending when the leaves are dry.
When two rain events are interrupted by a non-wet period, there are 3 possible scenarios:
- The non-wet period is shorter than 24 hours and the relative humidity is above 85%: all wet and non-wet hours are added.
- The non-wet period is shorter than 24 hours and the relative humidity is below 85%: only the wet hours are added.
- The non-wet period is greater 24 hours: the 2 wet periods are considered as two infection periods.
Because ascospore ejections during the night are low, when a leaf wetness period starts at night, hours of leaf wetness are calculated from sunrise only, except in the case of a massive ejection period or in a high inoculum orchard.
Minimum Temperature for Infection at Different Wetness Durations
| Wetness duration (hrs) | Temperature (°C) |
|---|---|
| 41 | 1 |
| 35 | 2 |
| 30 | 3 |
| 28 | 4 |
| 21 | 5 |
| 18 | 6 |
| 15 | 7 |
| 13 | 8 |
| 12 | 9 |
| 11 | 10 |
| 9 | 11 |
| 8 | 12-13 |
| 7 | 14-15 |
| 6 | 16-24 |
| 8 | 25 |
| Example - If wetness duration is 15 hrs. and mean temperature is less than 7°C during this period, there is no risk of infection. | |
Ascospore ejection period
Typically, the first mature ascospores (ready to cause an infection) are observed at green tip stage. Ascospore maturation then continues over a period of six to eight weeks. Generally, the largest quantity of mature ascospores available for ejection occurs during the tight cluster fruit-set stages.
The quantity of ascospores is limited (based on the level of scab in the previous fall) but maturation speed is mainly determined by temperature. Several models have been developed to predict the proportion of ascospores that will be ejected based on the accumulation of degree-days.
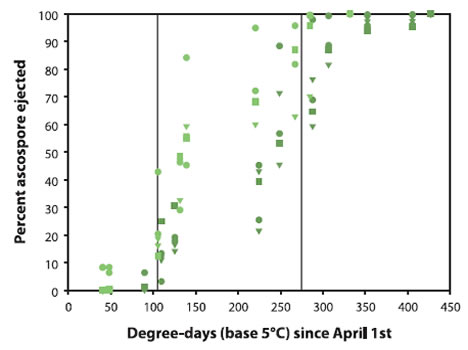
Description of above image
The following data is approximate.
| Degree days (base 5°C) since April 1st | Percent ascospore ejected |
|---|---|
| 40.5 | 0 |
| 8.3333 | |
| 0 | |
| 0 | |
| 0.0157 | |
| 48.15 | 6.4092 |
| 8.3652 | |
| 0.8938 | |
| 0.8938 | |
| 0.0157 | |
| 105.7 | 42.8782 |
| 20.4349 | |
| 18.7514 | |
| 16.2921 | |
| 12.6003 | |
| 131.5 | 46.3376 |
| 29.1172 | |
| 32.6736 | |
| 46.9774 | |
| 48.5134 | |
| 139.1 | 84.172 |
| 45.2729 | |
| 56.0935 | |
| 59.3454 | |
| 55.1451 | |
| 220.45 | 94.8958 |
| 72.1604 | |
| 68.9227 | |
| 60.0264 | |
| 68.0322 | |
| 267.1 | 95.7303 |
| 81.786 | |
| 87.5677 | |
| 62.8609 | |
| 86.8604 | |
| 284.7 | 99.6246 |
| 99.2573 | |
| 99.7585 | |
| 70.222 | |
| 95.6937 | |
| 331.85 | 100 |
| 100 | |
| 100 | |
| 100 | |
| 100 |
Degree-days calculation
Starting on April 1st, degree-days (DD) are calculated using the maximum temperature (Tmax) and the minimum temperature (Tmin) of each day integrated in the following formula; where the base temperature (Tbase) chosen is 5°C:
DD = [(Tmax + Tmin) ÷ 2] − Tbase
Ascospore maturation occurs in three phases identified by tree growth stage and the cumulated degree-days.
- Stage 1: green tip stage to tight cluster; ascospores mature slowly because temperature is usually cooler.
- Stage 2: from tight cluster to petal fall-first cover; maturation accelerates with increasing temperatures.
- Stage 3: the final phase of ascospore maturation, which ends when nearly all of the season's ascospores have been ejected, which is approximately two weeks after fruit-set on 'McIntosh'.
In practice, there is so much variation in the proportion of mature ascospores between leaves and among cultivars that degree-day models should be used only to determine the high risk period of ejection, from about 50 to 400 DD in base 5°C accumulated since April 1st.
Ascospores are released in the air during rain events or very heavy dew. Ascospores are ejected mostly during daytime even though some ascospores can be ejected during the night in orchards that were highly infected by apple scab the previous season.
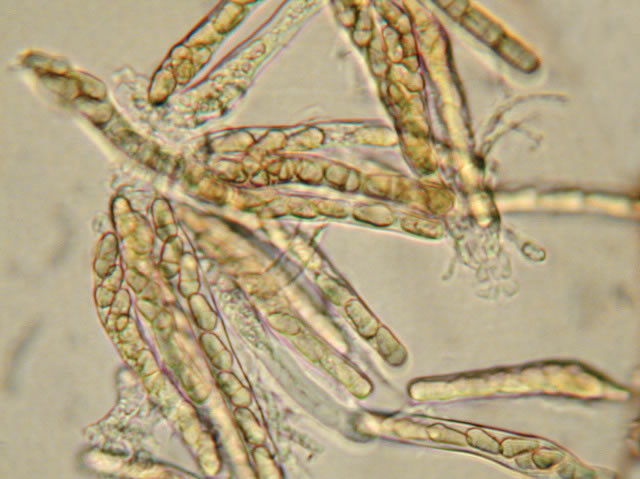
Ascospores of Venturia inaequalis in asci.
Leaf emergence: Estimation of the number of leaves per shoot
How to determine the number of leaves
The emergence of leaves in the spring is determined by temperature. It is possible to predict the emergence of new leaves based on the accumulation of degree-days. The tables below can be used to estimate the number of leaves on cluster or terminal shoots.
The degree-days must be calculated in base 5°C and accumulated from April 1st as:
Degree-Days = [(Tmax + Tmin) ÷ 2)] − 5°C
| Degree-Days | Number of Leaves |
|---|---|
| 96 | 1 |
| 110 | 2 |
| 120 | 3 |
| 130 | 4 |
| 140 | 5 |
| 154 | 6 |
| 178 | 7 |
|
Note: the accuracy in predicting the number of leaves per shoot is ±2 degree-days. |
|
| Degree-Days | Number of Leaves |
|---|---|
| 120 | 1 |
| 133 | 2 |
| 148 | 3 |
| 166 | 4 |
| 184 | 5 |
| 206 | 6 |
| 230 | 7 |
| 254 | 8 |
| 282 | 9 |
| 314 | 10 |
| 348 | 11 |
| 386 | 12 |
| 430 | 13 |
| 478 | 14 |
| 534 | 15 |
| 598 | 16 |
| 674 | 17 |
| 766 | 18 |
| 882 | 19 |
| 1033 | 20 |
|
Note: the accuracy in predicting the number of leaves per shoot is ±2 degree-days. |
|
Summary
Timing fungicide sprays: Primary infections
There are several factors to consider when deciding if a fungicide spray is needed to control a primary infection. Among them are:
- cultivar susceptibility;
- amount of inoculum in the orchard;
- weather conditions for ascospore maturation, ejection, and for infection;
- tree growth and emergence of leaves;
- efficacy of fungicides.
Previous fall
Determine if the potential amount of primary inoculum is low or high based on the sampling plan in method I or simplified method II (p. 6). If the amount of inoculum is expected to be high, fungicide protection against all infection periods will be needed.
Spring: Infection risk associated with the pathogen
- Measure or obtain daily data on rain (duration in hours) and temperature (°C). If possible, look at the weather forecast for the next few days.
- Calculate DD since April 1st:
DD = [(Tmax + Tmin) ÷ 2] − 5 °C - Determine if an infection period is forecasted (see table: Minimum Temperature for Infection at Different Wetness Durations).
- Optional: determine if the infection period is light, moderate or severe (see table below).
- Determine the potential for ascospore ejection during the next forecasted rain based on DD accumulation (see figure: Percent of ascospores ejected by degree-days (base 5°C) since April 1st). The greater the number of degree-days accumulated since the last ascospore ejection period, the greater is the risk of infection.
| Temperature (°C) | Severity of Infection | ||
|---|---|---|---|
| Light (hrs.) | Moderate (hrs.) | Heavy (hrs.) | |
| 25.5 | 10 | 14 | 23 |
| 25 | 8 | 11 | 18 |
| 24-25 | 6.5 | 9 | 16 |
| 16-24 | 6 | 9 | 16 |
| 15.5 | 6.5 | 10 | 17 |
| 14-15 | 7 | 11 | 19 |
| 12-13 | 8 | 12 | 20 |
| 11 | 9 | 14 | 22 |
| 10 | 11 | 16 | 26 |
| 9 | 12 | 17 | 27 |
| 8 | 13 | 21 | 34 |
| 7 | 15 | 23 | 37 |
| 6 | 18 | 27 | 44 |
| 5 | 21 | 34 | 50 |
| 4 | 28 | 42 | 57 |
| 3 | 30 | 52 | 65 |
| 2 | 35 | 69 | 93 |
| 1 | 41 | 69 | 93 |
Spring: Infection risk associated with apple tree growth
- Determine the number of new leaves since the last spray per cluster and terminal shoot based on degree-days accumulation (see tables: Cluster Shoots and Terminal Shoots).
- Spray according to forecast infection risk, ascospore ejection and amount of unprotected new leaves.
The efficacy of fungicide treatments will depend on:
- Leaf growth: new leaves are unprotected and leaf growth causes a 'fungicide dilution'.
- Rain intensity: protectant fungicides are washed off by 25 or more mm of rain.
- Time elapsed since the last spray: efficacy of fungicides decreases over time to reach the 'no efficacy level' after 7 to 10 days.
See example of risk estimation below:
| April 29 80 DD | May 4 102 DD | May 9 129 DD | May 14 162 DD | May 19 215 DD | May 24 259 DD | May 29 307 DD | June 3 342 DD | |
|---|---|---|---|---|---|---|---|---|
| Cluster | 0 | 0 | 4 | 6 | 7 | 7 | 7 | 7 |
| Terminal | 0 | 0 | 1 | 3 | 6 | 8 | 10 | 11 |
| New Leaves | 0 | 0 | 5 | 4 | 4 | 2 | 2 | 1 |
| Rain (hrs.) | 16 | 0 | 0 | 18 | 13 | 12 | 0 | 8 |
| Temperature (°C) | 8 | 14 | 18 | 14 | 9 | 14 | 15 | 19 |
| Infection | Yes | No | No | Yes | Yes | Yes | No | Yes |
| Ascospore Ejection | Yes | No | No | Yes | Yes | Yes | No | Yes |
| Risks* | P | A | A | H | H | P | A | H |
| Risks: A = absent, P = present; H = high | ||||||||
Summer
Secondary infections: Estimation of risks
Secondary infections or summer scab are caused by spores (conidia) that will increase in number with each uncontrolled primary infection and each new secondary infection. Hence, it is very important to avoid as much as possible secondary scab because the situation can quickly become out of hand. The best way to avoid summer scab is to efficiently control primary infections. Severity of summer infection also depends on temperature and duration of leaf wetness.
The following table provides the number of hours of leaf wetness required at different temperatures to cause a secondary infection.
Under humid or rainy and warm conditions, lesions may appear within one week after infection. In this situation, secondary scab will develop rapidly. On the other hand, if weather conditions are dry and hot, lesions will take more time to appear and may die and not produce a new generation of spores.
If there is poor control of primary infections, the number of fungicide sprays (and cost) could increase significantly during the summer months, depending on prevailing weather conditions.
It is difficult to determine if summer sprays are needed. A very small amount of scab at the end of the primary infection period could result in unacceptable fruit scab at harvest (1 to 2%) if the summer is rainy, but it is not possible to know in advance if the summer will be rainy. Thus, it is advisable to use a conservative threshold for determining the need for summer sprays. An experiment conducted in Quebec on 'McIntosh' showed that this threshold is 0.05 scabbed leaves per shoot at the end of the primary infection period. Note: this threshold has been tested in Quebec only.
| Wetness duration (hrs.) | Temperature (°C) |
|---|---|
| 12 | 9 |
| 11 | 10 |
| 9 | 11 |
| 8 | 12-13 |
| 7 | 14-15 |
| 6 | 16-24 |
| 8 | 25 |
Summer Scab: Spraying or not spraying
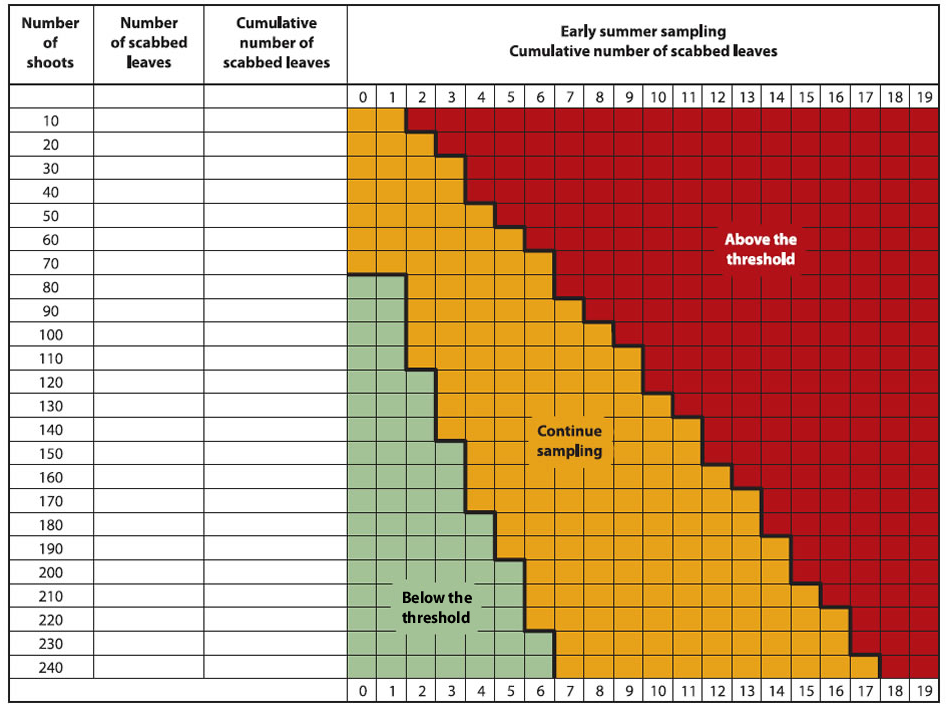
Growers are advised to inspect their orchard when nearly all ascospores are predicted to be ejected and all potential primary scab lesions have appeared. It is recommended to look at shoots that are representative of the orchard block and the trees, making sure to include the shoots at the top of the trees, as they often have lesions. Considering that it is difficult to detect low level of scab, such as 0.05 scabbed leaves per shoot, a sequential plan has been developed (Figure below).
Sequential Sampling Procedure
- Count the number of scabbed leaves on a minimum of 80 shoots randomly chosen in the orchard (both cluster and terminal shoots).
- If the number is below the threshold (green zone), the recommended summer scab fungicide program may be reduced or eliminated.
- If the number of leaves is above the threshold (red zone), the full recommended fungicide program will be needed during all summer.
- If the number of scabbed leaves is in the intermediate zone (orange zone), sampling must continue (another series of 10 shoots) before a decision can be made.
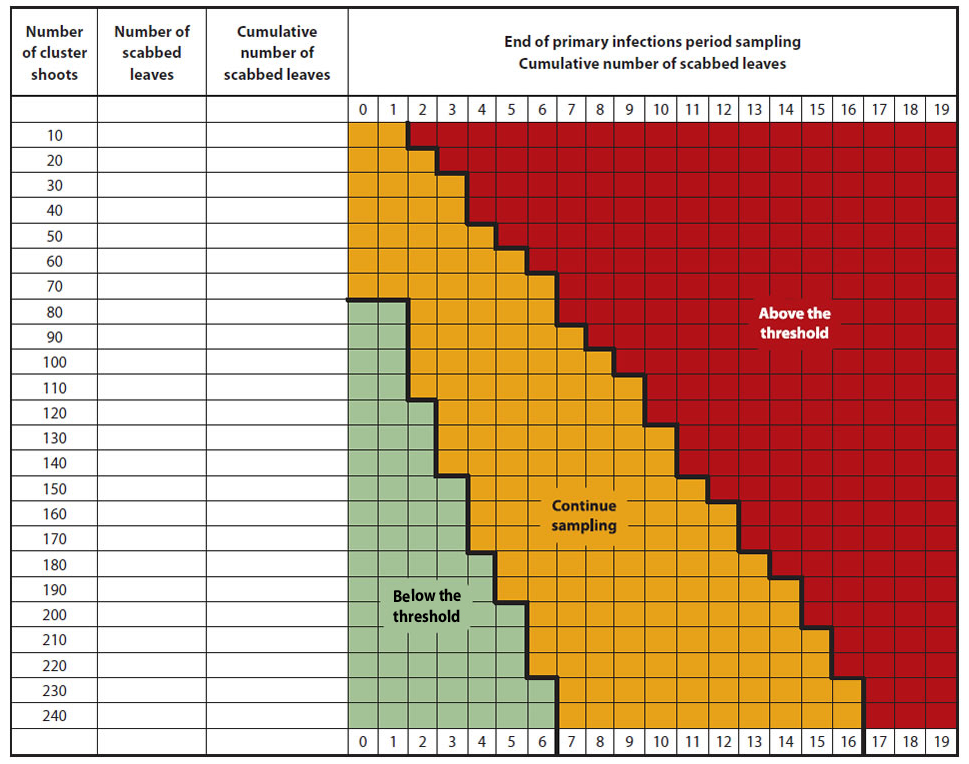
Description of the above image
| Number of shoots | Number of scabbed leaves | Cumulative number of scabbed leaves | End of primary infections period sampling - Cumulative number of scabbed leaves | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | |||
| 10 | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 20 | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 30 | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 40 | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 50 | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 60 | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 70 | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 80 | B | B | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 90 | B | B | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 100 | B | B | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | ||
| 110 | B | B | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | ||
| 120 | B | B | B | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | ||
| 130 | B | B | B | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | ||
| 140 | B | B | B | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | ||
| 150 | B | B | B | B | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | ||
| 160 | B | B | B | B | C | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | ||
| 170 | B | B | B | B | C | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | ||
| 180 | B | B | B | B | B | C | C | C | C | C | C | C | C | C | A | A | A | A | A | A | ||
| 190 | B | B | B | B | B | C | C | C | C | C | C | C | C | C | C | A | A | A | A | A | ||
| 200 | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | A | A | A | A | A | ||
| 210 | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | C | A | A | A | A | ||
| 220 | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | C | A | A | A | A | ||
| 230 | B | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | C | A | A | A | ||
| 240 | B | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | C | A | A | A | ||
|
||||||||||||||||||||||
Note: If the maximum number of shoots sampled is reached (240 shoots) and a decision cannot be taken (yellow zone). Re-sample one week later.
Description of the above image
| Number of shoots | Number of scabbed leaves | Cumulative number of scabbed leaves | End of primary infections period sampling - Cumulative number of scabbed leaves | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | |||
| 10 | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 20 | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 30 | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 40 | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 50 | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 60 | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 70 | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 80 | B | B | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 90 | B | B | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 100 | B | B | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | ||
| 110 | B | B | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | ||
| 120 | B | B | B | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | ||
| 130 | B | B | B | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | ||
| 140 | B | B | B | C | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | ||
| 150 | B | B | B | B | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | ||
| 160 | B | B | B | B | C | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | ||
| 170 | B | B | B | B | C | C | C | C | C | C | C | C | C | C | A | A | A | A | A | A | ||
| 180 | B | B | B | B | B | C | C | C | C | C | C | C | C | C | A | A | A | A | A | A | ||
| 190 | B | B | B | B | B | C | C | C | C | C | C | C | C | C | C | A | A | A | A | A | ||
| 200 | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | A | A | A | A | A | ||
| 210 | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | C | A | A | A | A | ||
| 220 | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | C | C | A | A | A | ||
| 230 | B | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | C | A | A | A | ||
| 240 | B | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | C | C | A | A | ||
|
||||||||||||||||||||||
Chemical control
Mode of action of fungicides
Elements of successful chemical management
- Timing of fungicides based on infection risk, time elapsed since the last spray and weather forecast
- Choice of fungicides based on level of infection risk and anti-resistance management
- The proper dose, mix and application
Types of fungicides
There are 2 types of fungicides registered against apple scab in Canada:
- Those which are not absorbed by the plant and which affect spore germination (protectant).
- Those which are absorbed by the plant and affect fungal growth (curative).
Modes of intervention
Fungicides can be used according to two modes of intervention. They are pre-infection and post-infection.
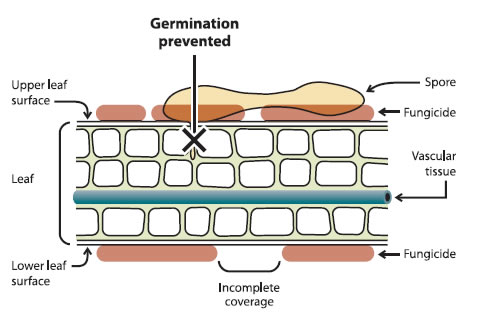
A pre-infection fungicide is applied before an infection period begins, and protects the leaves and fruits from becoming infected. The product will be effective only on the leaves or fruits present when the trees are sprayed. The active ingredient prevents the first stages of fungus development, e.g. spore germination (Figure 1). Example of pre-infection fungicides: Captan and Dithane.
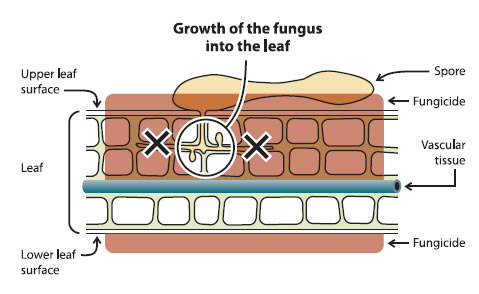
Post-infection fungicides prevent the fungus from colonizing host tissue (Figure 2). This type of intervention provides control when the fungicide is applied after an infection period has begun, when too much weathering of the previous pre-infection spray has occurred, or in cases of severe infection. Example of post-infection fungicides: Nova, Flint, Nustar, Scala, Vangard and Sovran.
Figure 1 - Mode of action of fungicide used in a pre-infection application
Description of the above image
A leaf that has been sprayed with pre-infection fungicide has incomplete coverage on the upper and lower leaf surfaces however it still helps prevent spores from germinating in the host and vascular tissue of the leaf.
Figure 2 - Mode of action of fungicide used in post-infection
Description of the above image
A leaf that has been treated with post-infection fungicide has complete coverage on the upper and lower leaf surfaces as well as in the host tissue of the leaf, however, the growth of the fungus into the leaf from the spore remains.
Fungicide modes of action
Fungicides can be divided in two categories: unisite and multisite fungicides.
Unisite fungicides target a specific function of fungal development, and this opens the door to resistance development. Just one mutation on the target site or any other means of avoiding or countering the effect of the fungicide can lead to a significant loss of efficacy of the fungicide. Biosynthesis of compounds essential to the development of the fungus, respiration and cellular division are the most common targets of unisite fungicides.
Multisite fungicides act on several functions of fungal development. They are less prone to resistance development because mutations in the fungus must occur at all target sites for resistance to develop. Most contact fungicides like Dithane, Captan and Polyram, and elemental fungicides like copper and sulfur that are applied pre-infection, are members of this category.
Development of fungicide resistance
There are several possible reasons why a fungicide fails to control apple scab: spray operator error or poor sprayer calibration, excessive wind or wash off by rain, or resistance of the pathogen to the fungicide. Fungicide resistance is often not the reason for scab control failure but does occur.
Fungicide resistance is a natural phenomenon in which some members (spores) of the fungal population are capable of resisting the detrimental effect of fungicides. These individuals will transmit their resistance to their progeny after being subjected to several fungicide sprays. The proportion of resistant individuals may reach high levels leading to disease control failure. The number of sprays necessary to achieve a shift in control efficacy varies according to fungicide use and initial composition of the population when the fungicide is first used.
The loss of sensitivity of a fungus to a fungicide can occur according to two scenarios. In one scenario, a sudden decline of sensitivity leads to a fast and almost irreversible loss of efficacy, regardless of the applied rate. This has occurred with the benzimidazoles Benlate and Senator.
In the other scenario, the loss of efficacy may be gradual, as it is observed for sterol inhibitors like Nova and Nustar. Resistance mechanisms help the fungus prevent or avoid partially the effect of the fungicide. Disease control is dose-dependant, so the application of a half-dose will increase the selection of less-sensitive individuals.
Fungicide efficacy
Fungicide Resistance: Current Situation in Quebec Orchards
Fungicide resistance is an important factor to consider in rational apple scab management, and anti-resistance strategies are essential, regardless of the situation in the orchard. A study recently conducted by Agriculture and Agri-Food Canada in a great number of orchards in Quebec reported a general insensitivity of fungal isolates to the demethylation inhibitors (DMI) Nova and Nustar.
Most growers still use DMIs without major loss of disease control. Nevertheless, the study shows a potential for resistance to occur if these fungicides continue to be used in these orchards, so these results deserve attention.
Proportion of samples insensitive to Nova: 70% Footnote 1Footnote 3
Strobilurins, represented by the fungicides Sovran and Flint, were also evaluated in the study conducted in Quebec orchards. Even though some isolates displayed a reduced sensitivity to the active ingredient, it has been demonstrated that this resistance mechanism is not active in the presence of the host plant.
Strobilurin resistance cases are common in cereal crops and are also reported in some orchards in Europe, so we must keep our eyes open in apple orchards.
The fungicide dodine can also be an option in a scab management scheme, although many apple growers in the northeast United States stopped applying dodine due to resistance development. The Quebec study showed that dodine is still effective in the majority of orchards sampled, but we have to remain cautious since the history in the US clearly showed the link between dodine use and the development and persistence of dodine resistance. If dodine sprays were frequent in the past decades in a given orchard, the risk is still high.
Proportion of samples insensitive to Dodine: 34% Footnote 2Footnote 3
Management of fungicide resistance
It is crucial to include in your scab management scheme some practices aimed at slowing down or stopping fungicide resistance development. Given the small number of fungicides available for post-infection control, the loss of one fungicide is not a viable option, as it will deprive growers of one more tool in a decreasing number of alternative families of fungicides.
The use of fungicides with different modes of action remains the most effective way of slowing down the continual selection of insensitive isolates toward one particular fungicide. Thus, consecutive application of the same post-infection fungicide or of two fungicides belonging to the same chemical family is not recommended.
The application of these fungicides at full label rate is also very important. The exposure of the fungus to half-rates favors the selection of less sensitive isolates, increasing their proportion within the population.
Do not forget that unisite fungicides are more prone to fungicide resistance development. Multisite fungicides like Captan, Dithane and Polyram are less affected by resistance build-up due to their mode of action.
Beware of slightly declining levels of control that could be linked to the emergence of resistance in your orchard, but remember that many other factors come into play when managing apple scab.
Sanitation treatments and the application of a biocontrol agent that reduce the primary inoculum (ascospores) will help delay the buildup of resistance to fungicides, and may allow a reduction in fungicide usage, particularly in low-risk orchards.
Benzimidazoles, including Senator, also form one family of fungicide, but their efficacy is largely compromised due to resistance occurrence.
Families of fungicides prone to resistance
- DMI
Nova, Nustar - Strobilurins
Sovran, Flint - Anilinopyrimidins
Vangard, Scala - Guanidine
Dodine
Sequential sampling: Collection data sheet template
End of primary infections
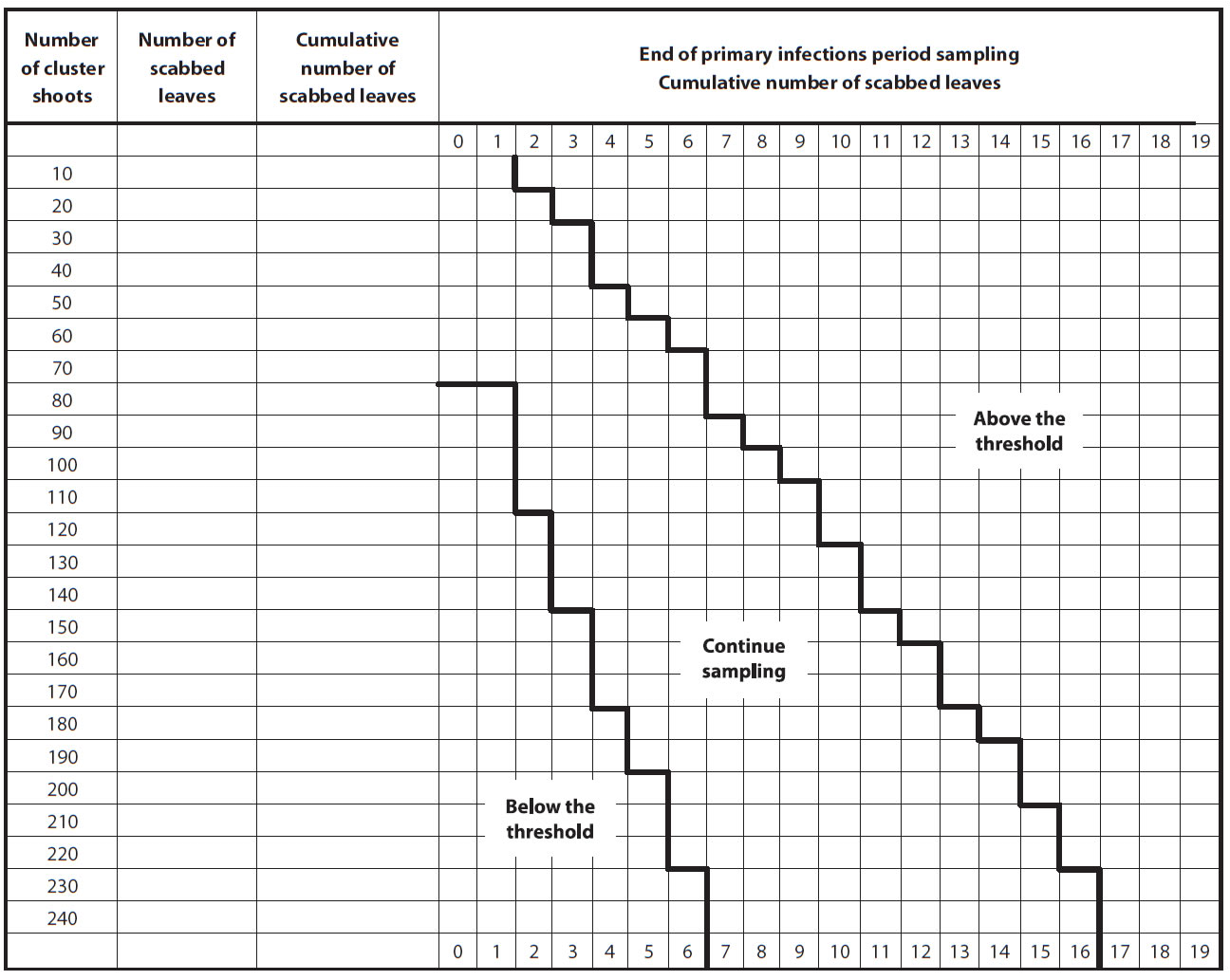
Description of above image
| Number of shoots | Number of scabbed leaves | Cumulative number of scabbed leaves | End of primary infections period sampling - Cumulative number of scabbed leaves | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | |||
| 10 | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 20 | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 30 | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 40 | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 50 | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 60 | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 70 | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 80 | B | B | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 90 | B | B | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 100 | B | B | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | ||
| 110 | B | B | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | ||
| 120 | B | B | B | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | ||
| 130 | B | B | B | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | ||
| 140 | B | B | B | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | ||
| 150 | B | B | B | B | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | ||
| 160 | B | B | B | B | C | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | ||
| 170 | B | B | B | B | C | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | ||
| 180 | B | B | B | B | B | C | C | C | C | C | C | C | C | C | A | A | A | A | A | A | ||
| 190 | B | B | B | B | B | C | C | C | C | C | C | C | C | C | C | A | A | A | A | A | ||
| 200 | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | A | A | A | A | A | ||
| 210 | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | C | A | A | A | A | ||
| 220 | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | C | A | A | A | A | ||
| 230 | B | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | C | A | A | A | ||
| 240 | B | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | C | A | A | A | ||
|
||||||||||||||||||||||
Early summer
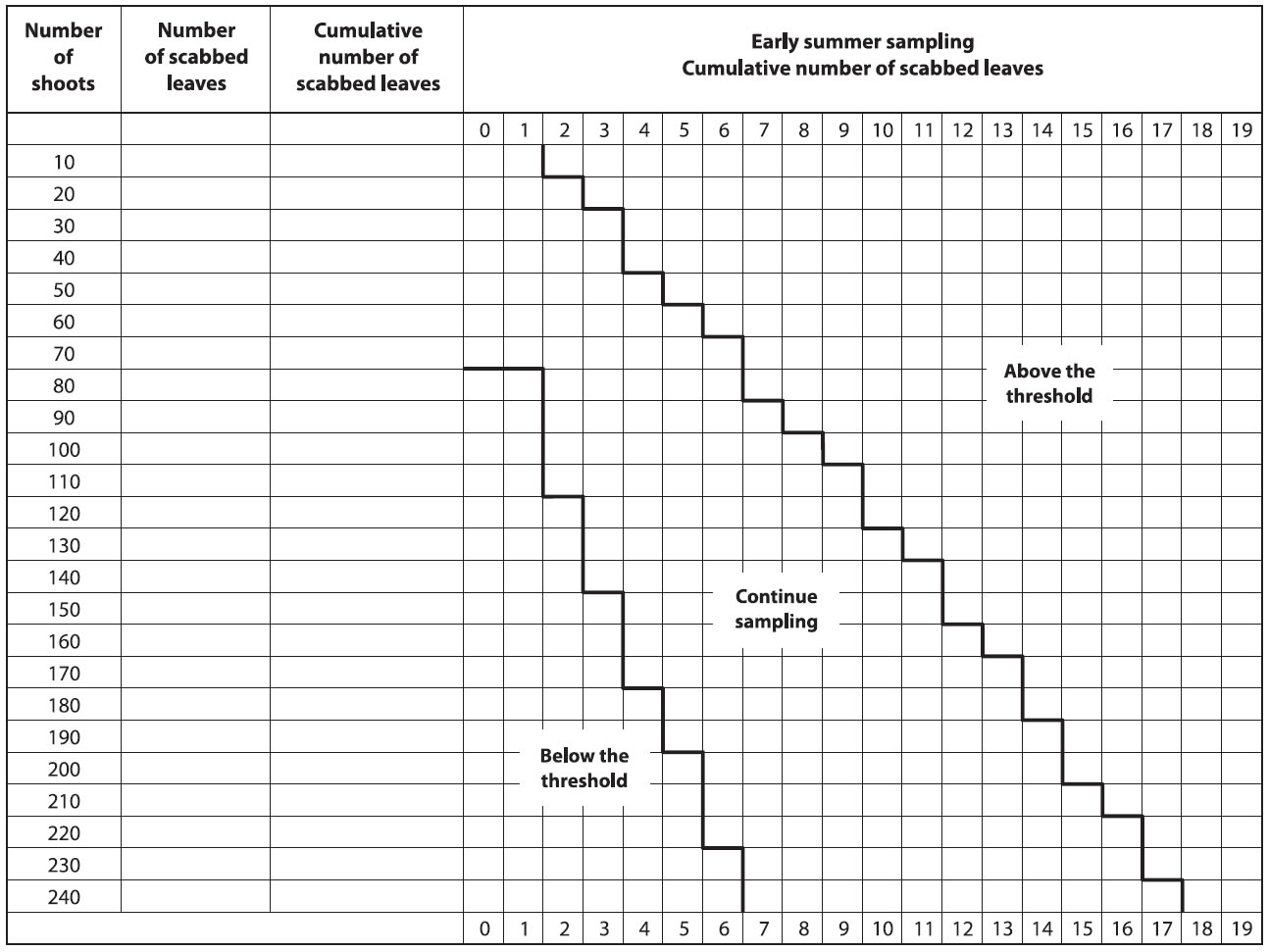
Description of above image
| Number of shoots | Number of scabbed leaves | Cumulative number of scabbed leaves | End of primary infections period sampling - Cumulative number of scabbed leaves | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | |||
| 10 | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 20 | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 30 | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 40 | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 50 | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 60 | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 70 | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 80 | B | B | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 90 | B | B | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | A | ||
| 100 | B | B | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | A | ||
| 110 | B | B | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | ||
| 120 | B | B | B | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | A | ||
| 130 | B | B | B | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | A | ||
| 140 | B | B | B | C | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | ||
| 150 | B | B | B | B | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | A | ||
| 160 | B | B | B | B | C | C | C | C | C | C | C | C | C | A | A | A | A | A | A | A | ||
| 170 | B | B | B | B | C | C | C | C | C | C | C | C | C | C | A | A | A | A | A | A | ||
| 180 | B | B | B | B | B | C | C | C | C | C | C | C | C | C | A | A | A | A | A | A | ||
| 190 | B | B | B | B | B | C | C | C | C | C | C | C | C | C | C | A | A | A | A | A | ||
| 200 | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | A | A | A | A | A | ||
| 210 | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | C | A | A | A | A | ||
| 220 | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | C | C | A | A | A | ||
| 230 | B | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | C | A | A | A | ||
| 240 | B | B | B | B | B | B | B | C | C | C | C | C | C | C | C | C | C | C | A | A | ||
|
||||||||||||||||||||||
Acknowledgements
Reviewers
- Dr. R. Beresford, Scientist, BioProtection Group, HortResearch, Private Bag 92 169, Auckland, New Zealand.
- Dr. W.E. MacHardy, Professor of Plant Pathology (retired), Plant Biology Dept. 246 Spaulding Hall Univ. of New Hampshire Durham NH. U.S.A.
- Dr. L. Parisi, Scientist, UERI, Domaine de Gotheron, INRA d'Avignon, 26320 Saint-Marcel-lès-Valence, France.
- Dr. L. Berkett, Ph.D., Department of Plant and Soil Science, Hills Building, 105 Carrigan Drive, University of Vermont, Burlington, VT 05405.
- Sylvie Bienvenue, Technologist in agriculture, Centre Agricole Bienvenue Inc.
- Thérèse Otis, agr., Manager of Science Creative and Publishing Services, Agriculture and Agri-Food Canada, Saint-Jean-sur-Richelieu.
Partners
- Fédération des producteurs de pommes du Québec
- Ontario Apple Growers
Conception
Nora-Audrey Carisse-Landry
Funding
Pest Management Centre, Agriculture and Agri-Food Canada
Graphic Design and Page Layout
- Natacha Sangalli, Graphic designer, Science Creative and Publishing Services, Agriculture and Agri-Food Canada (Saint-Jean-sur-Richelieu).
- Mike Shillinglaw, Graphic designer, Science Creative and Publishing Services, Agriculture and Agri-Food Canada (Winnipeg).