I. Introduction
Botrytis leaf blight (BLB) of onion, caused by the fungal pathogen Botrytis squamosa Walker, is an important disease that threatens onion production in Canada and other onion production areas of the world. The disease is endemic and is especially severe on yellow globe onions.
Onion growers are concerned about adequate management of this disease in their crops as no commercially available onion cultivars are fully resistant to B. squamosa. Moreover, some of the fungicides used for this disease are either under regulatory re-evaluation (iprodione) or already phased out (vinclozoline), and there is a risk of pathogen populations developing resistance to current fungicides. Resistance of B. squamosal isolates to some of the older fungicides (for example dicarboximides) is already reported.
Agriculture and Agri-Food Canada's Pest Management Centre has funded work to develop new decision support tools and information which can be used in an integrated system for management of BLB. This factsheet summarizes key tools and approaches recommended as a result of this work. These tools can help growers achieve improved control of BLB and judicious use of fungicides, while reducing crop protection costs and optimizing fungicide resistance management.
Botrytis leaf blight of onion develops in two phases. It starts with a leaf spotting phase followed by a leaf blighting phase. Initially, spot lesions appear on leaves 24-48 hours after an inoculation and are whitish in color, 1 to 5 mm in length and generally surrounded by greenish-white halos which appear water-soaked at first (Figure 1).
As lesions age, the centres become sunken, straw coloured and sometimes develop a slit that is oriented lengthwise in the lesion. Older onion leaves are more susceptible than younger leaves. Partial or complete leaf blighting generally occurs within 5 to 12 days after the initial lesion development (Figure 2). Under severe epidemics, the entire crop may express advanced blight symptoms (Figure 3).

Figure 1: Discrete spot lesion caused by Botrytis squamosa on an onion leaf.

Figure 2: Upper leaf blighting symptom caused by Botrytis squamosa.

Figure 3: Onion crop severely infected by Botrytis squamosa.
II. Epidemiology
Botrytis squamosa overwinters as sclerotia (compact mass of fungal mycelium) formed on infected onion leaves and necks of onion bulbs that remain in the soil after harvest or on crop debris in cull piles. In the spring, ascospores produced within apothecia arising from sclerotia (Figure 4) are released in the air infecting leaves of new onion plants. However, ascospores are not considered to be a significant source of primary inoculum.

Figure 4: Apothecia arising from a Botrytis squamosa sclerotium.

Figure 5: Conidia produced on a Botrytis squamosa sclerotium.
Epidemics are mainly initiated by conidia, a spore type produced on sclerotia overwintering in soil, on onion debris or on onion cull piles (Figure 5). Sclerotia of B. squamosa can produce conidia over a prolonged period of time, usually extending from spring to early summer.
The optimum condition for conidia to infect onion leaves is a combination of air temperatures between 18-20˚C and leaf wetness for at least 6 hours (Figure 6). Following the leaf spotting phase of the disease, B. squamosa continues to colonize the infected leaves causing blighting and die back. New sporulation and subsequent conidia production occurs on dead leaves, mainly around the dead leaf tip (Figure 7). This occurs mainly at night, when the leaves are wet for at least 12 hours and the mean temperature is between 8 and 22°C.

Figure 6: Morning dew on a young onion leaf.

Figure 7: Conidia produced on dead onion leaves.
In the spring, temperature is generally not a limiting factor for the production of conidia because these are produced at temperatures ranging from 3 to 27˚C (optimum of 9˚C). The initial cohorts of conidia developing from sclerotia are responsible for the leaf spotting phase of the disease. At this stage, the disease generally progresses slowly. However, as leaf blighting and leaf tip dieback progress, new crops of conidia are produced on dead tissues which are capable of initiating subsequent disease cycles. At this stage, disease spreads much faster and wider across onion crops.
III. Disease management
The conventional fungicide spray program for onion leaf blight management comprises application of fungicides at a fixed 7 to 10 day interval from the 3-4 leaf growth stage to shortly after the onions lodge. In eastern Canada, growers typically start their fungicide programs with a preventive fungicide, such as a dithiocarbamate applied at 7 to 10 day intervals, followed by fungicides such as chlorothalonil or iprodione often mixed with a dithiocarbamate, depending on disease pressure. Mixtures of fungicides are also used to provide control for both botrytis leaf blight and downy mildew (Perenospora destructor). The fungicides Pristine (pyralostrobin and boscalid, BASF) and Switch (cyprodonil and fludioxonil, Syngenta) are incorporated when disease pressure is perceived to be high.
a. Limitations of fixed interval fungicide spray programs
A common fixed interval fungicide program for Botrytis leaf blight may involve 6 to 14 fungicide sprays per season, among which are 3 or 4 sprays with iprodione applied alone or in a fungicide mixture. This intensive spray program can negatively impact the sustainability of BLB management. Pathogen insensitivity to fungicides can be a serious problem.
Frequent and repetitive use of fungicides of similar groups and modes of action in particular can lead to resistance development in the pathogen population. Laboratory tests comparing the sensitivity of field isolates of B. squamosa to currently used fungicides revealed that all isolates were resistant to mancozeb (Dithane DG) and chlorothalonil (Bravo 500), but a few isolates were insensitive to both dicarboximide fungicides iprodione (Rovral) and vinclozolin (Ronilan) (Figure 8).
Another limitation is the production cost, which often increases with the overuse of fungicides, especially when disease pressure does not reach economic thresholds and spray applications are deemed unwarranted.
Figure 8. Incidence of Botrytis squamosa isolates resistant to fungicide iprodione monitored from 2000 to 2004 in the main onion production region in the province of Quebec varied from 8 to 21%.
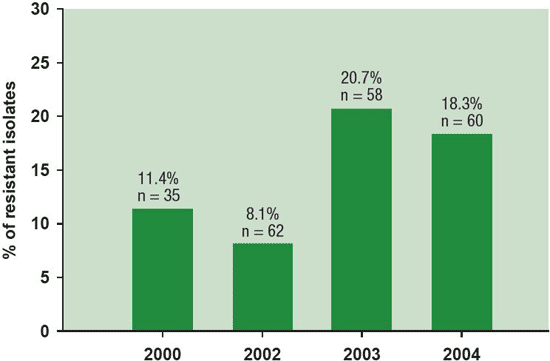
Description - Figure 8
% of resistant isolates
n = number of resistant Botrytis squamosa isolates tested each year
- 2000 - 11.4%, n=35
- 2002 - 8.1%, n=62
- 2003 - 20.7 %, n=58
- 2004 - 18.3%, n=60
In recent years, new fungicides belonging to different chemical groups were registered for management of BLB, including boscalid (Lance or Endura, BASF), boscalid and pyraclostrobin (Pristine, BASF), and cyprodinil and fludioxonil (Switch, Syngenta). The availability of these fungicides allows for a better rotation of products with different modes of action (fungicide groups), which in turn leads to better resistance management.
b. Estimating disease risk
The potential yield losses due to BLB vary widely (for example 7 to 30%) depending on weather conditions and disease pressure during the season. Therefore, the frequency and timing of fungicide sprays may be crucial in some years and less important in other years. In years with low rainfall, BLB may be so low that there is no need to apply fungicides for disease control. However, in most years, humidity is high and leaves need to be protected from disease to avoid yield loss and to ensure that plants have sufficient green leaves to absorb the sprout inhibitor applied to onions intended for long term storage.
Determining the risk of BLB, the need for, and the optimum timing of fungicide applications in onion crops can improve disease control while avoiding unnecessary sprays, thus saving on treatment costs. There are a number of disease prediction models proposed to date to estimate the risk of BLB development in onion crops. Some predictors rely on monitoring-based risk indicators derived from field observations, some others on weather-based risk indicators related to conditions conducive to disease development. A recent study showed that monitoring-based predictors are more reliable; however some weather-based predictors have provided good estimation of disease risk. Table 1 summarizes the findings from this study, indicating the performance levels of various BLB predictors.
Good disease forecasting and management decision support tools can improve control efficacy with less fungicide use, which in turn can slow down or prevent the development of fungicide insensitivity and can lower crop protection costs.
Table 1.a Levels of prediction accuracy and reliability of various Botrytis leaf blight disease risk indicators: Monitoring-Based
| Disease risk indicators | % of good decisions - First spray |
% of good decisions - Subsequent sprays |
|---|---|---|
| Airborne inoculum | 89 | 82 |
| Number of lesions on oldest leaves | 85 | 82 |
| Number of lesions on youngest leaves | 82 | 75 |
Table 1.b Levels of prediction accuracy and reliability of various Botrytis leaf blight disease risk indicators: Weather – Based
| Disease risk indicators | % of good decisions - First spray |
% of good decisions - Subsequent sprays |
|---|---|---|
| Sporulation Index | 64 | 73 |
| Inoculum Production Index | 72 | 63 |
| Infection Probability | 36 | 57 |
| Disease Severity Values | 67 | 71 |
c. Knowledge-based management: A stepwise decision making process
Optimum management of BLB should be based on regular field observations, including monitoring of airborne inoculum present and number of lesions per leaf (thresholds established for BLB are 3 lesions on the oldest leaves and 1 lesion on the youngest leaves), as well as past, present and forecasted weather conditions (Figure 9). Generally, field observations are more costly to obtain than regional weather conditions.
Figure 9. Key factors to determine risk for Botrytis leaf blight disease.
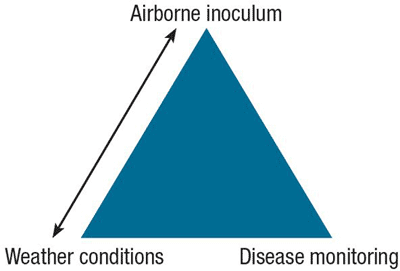
Weather conditions and airborne inoculum are linked because there is a correlation between them. Disease monitoring is important to confirm the situation in the field.
Optimum management of Botrytis leaf blight could be achieved following a stepwise approach:
Step 1. Using weather-based risk indicators. For eastern Canada, the best weather-based risk indicators are the Sporulation Index developed by Lacy and Pontius in 1983 and the Disease Severity Values proposed by Sutton et al., in 1986 (Figure 10). The Sporulation Index represents the degree of favourability of weather factors, mainly temperature and vapor pressure deficit, on the production of spores by B. squamosa on a scale of 0 to 100, where 0 represents no potential for sporulation and 100 conditions that are highly favorable for sporulation. Warning and action threshold of 50 and 80, respectively were set based on field experimentations. The cumulative disease severity value represents the progress of BLB. The best way to use these risk indicators is to combine the two indices calculated from weather forecasts.
Figure 10. Performance of weather-based risk indicators for predicting Botrytis leaf blight.
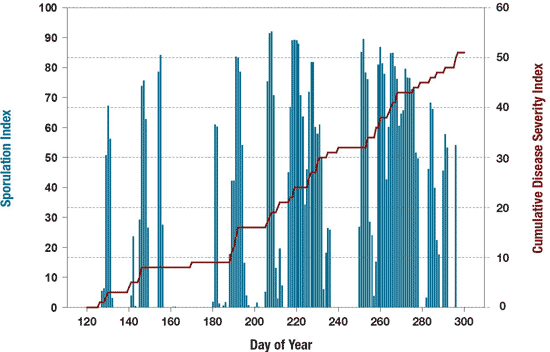
Description - Figure 10
| Day of Year | Minimum Value | Maximum Value |
|---|---|---|
| 120 | 3 | 68 |
| 140 | 0.5 | 82 |
| 160 | 0.1 | 0.2 |
| 180 | 2 | 81 |
| 200 | 0.1 | 92 |
| 220 | 6 | 81 |
| 240 | 0 | 85 |
| 260 | 45 | 81 |
| 280 | 0 | 69 |
| 300 | 0 | 0 |
| Day of Year | From | To |
|---|---|---|
| 120 | 0 | 6 |
| 140 | 6 | 8 |
| 160 | 8 | 9 |
| 180 | 9 | 14 |
| 200 | 14 | 21 |
| 220 | 21 | 31 |
| 240 | 31 | 32 |
| 260 | 32 | 42 |
| 280 | 42 | 45 |
| 300 | 45 | 51 |
Step 2. Monitoring airborne inoculum. The concentration of airborne inoculum (spores) of B. squamosa was identified as a good risk indicator for disease development. Concentration of spores can be measured by using a rotating-arm spore sampler such as the one shown in Figure 11. This information can be used to determine the timing for the initial spray and the need for subsequent applications (Table 1).

Figure 11. Rotating-arm spore sampler
Step 3. Disease monitoring. Knowing what is happening in the field is essential to optimize BLB management. Field monitoring of lesion development as well as disease incidence and severity could be used to reinforce the need for fungicide sprays as determined by disease predictors, airborne inoculum or both. In addition, BLB monitoring could be used to evaluate the effectiveness of the last fungicide sprays (Figure 12).
Several tools and new information are now available for optimal and sustainable use of fungicides to manage BLB of onion. Also, some of these tools and information are being offered to growers through various services available in some eastern provinces (for example Quebec and Ontario). Using these tools will enable growers to maintain disease pressure below the economic threshold and achieve optimal onion yield with minimum use of fungicides.
Figure 12. Relationship between airborne inoculum and BLB progress in a managed onion field.
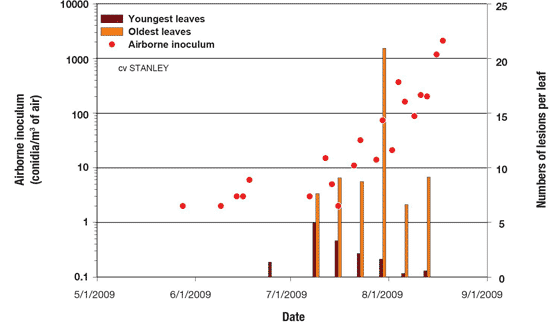
Description - Figure 12
| Date (yyyy-mm-dd) |
Airborne inoculum (conidia/m3 of air) Scale from 0.1 to 10,000 |
Numbers of lesions per leaf (Scale from 0 to 25) |
|---|---|---|
| 2009-06-01 | 13 | 0 |
| 2009-07-01 | 0 | 0 |
| 2009-08-01 | 800 | 22 |
The authors acknowledge the collaboration of fellow researchers David-Mathieu Tremblay and Audrey Levasseur (AAFC), Hervé Van der Heyden (Phytodata Inc.) and Dr. Mary Ruth McDonald (University of Guelph) in conducting the studies highlighted here and their contribution to this publication.
For more information about this project, please contact: Dr. Odile Carisse or Luc Brodeur
Other useful resources
- Carisse, O., McRoberts, N., and Brodeur, L. 2008. Comparison of monitoring- and weather-based risk indicators of Botrytis leaf blight of onion and determination of action thresholds. Canadian Journal of Plant Pathology 30: 442-456.
- Carisse, O., Tremblay, D.M., Lévesque, C.A., Gindro, K., Ward, P., and Houde, A. 2009. Development of a TaqMan real-time PCR assay for quantification of airborne conidia of Botrytis squamosa and management of Botrytis leaf blight of onion. Phytopathology 99: 1273-1280.
- Carisse, O., Tremblay, D.M., McDonald, M.R., Brodeur, L., and McRoberts, N. 2011. Management of Botrytis leaf blight of onion: the Quebec experience of 20 years of continual improvement. Feature Paper, Plant Disease 95: 204-514.
- Van der Heyden, H., Carisse, O., and Brodeur, L. 2011. Comparison of monitoring based indicators for initiating fungicide spray programs to control Botrytis Leaf Blight of onion. Crop Protection 33 : 21-28
- Carisse, O., Levasseur, A. and Van der Heyden, H. 2011. An improved risk indicator for Botrytis leaf blight of onion caused by Botrytis squamosa. Plant Pathology (in press).
About the Pesticide Risk Reduction at Agriculture and Agri-Food Canada
The Pesticide Risk Reduction team delivers viable solutions for Canadian growers to reduce pesticide risks in the agricultural and agri-food industry. The team achieves this goal by funding integrated pest management projects and coordinating pesticide risk reduction strategies developed through consultation with stakeholders and pest management experts. Other sustainable crop protection factsheets are available. For more information please visit the Pest Management Centre.