Apple scab is a serious disease caused by the pathogenic fungus Venturia inaequalis and managed primarily through multiple applications of conventional fungicides. In recent years, however, indications have been that some of these products have become less effective, likely due to the development of resistance.
In 2011, the Agriculture and Agri-Food Canada’s Pesticide Risk Reduction Program (PRRP) initiated a project aiming to develop testing protocols and document the spread of pathogen resistance to strobilurin and sterol inhibitor (SI) fungicides through a survey across apple growing regions in Canada. The results and the outcomes of this project are summarised in this factsheet.
This initiative is one of the actions being supported in the context of a Reduced-Risk Strategy for Apple Scab Management, led by the PRRP in collaboration with stakeholders. The goal of the strategy is to develop tools which could be combined into an integrated approach to manage this disease in a sustainable and cost-effective manner.
Biology, damage, and management of apple scab
Apple scab causes damage to foliage and fruit, often making the crop unmarketable. On leaves, young lesions are often not noticeable until after petal fall (Figure 1). With time, they turn dark brown to black with distinct margins. Lesions on young fruit resemble leaf symptoms, develop slowly, and become brown and corky, often resulting in uneven growth and cracking of the skin (Figure 2).

Figure 1: Young apple scab lesions on leaf are velvety brown to olive green with indistinct margins.
Photo credit: Ontario Ministry of Agriculture, Food and Rural Affairs.

Figure 2: Mature apple scab lesions on fruit are typically brown, corky, with cracking of the skin.
Photo credit: Ontario Ministry of Agriculture, Food and Rural Affairs.
The apple scab pathogen overwinters in infected leaves or fruit on the orchard floor. Maturation of ascospores (primary inoculum causing early season infection) starts as the tree emerges from dormancy (green tip) and peaks during late pink to early bloom. Initial infections lead to production of spores which can cause multiple secondary infections during the season. Wind and moisture are necessary for spore discharge and germination as well as for subsequent infection of apple tissue. With frequent rainfall, the control of apple scab becomes extremely difficult, particularly if the disease becomes established from primary infections in the spring.
Sterol inhibitor fungicides (Group 3), including Nova 40W, Nustar and Inspire, and strobilurin fungicides (Group 11), including Sovran, Flint 50 WG, and Pristine WG, have been used extensively in the last two decades. Having a site-specific mode of action, these classes of fungicides are at a higher risk for resistance development among pathogen populations. This risk is further increased by the multiple applications of these products over the growing season. Preliminary tests conducted in previous years suggested that resistance to strobilurin and SI fungicides exists among scab pathogen populations in certain Canadian orchards.
Resistance management practices based on specific knowledge of resistance prevalence in individual orchards could contribute to the longevity of registered products, resulting in economic benefits for growers and reduced risks from these pesticides.
Survey and resistance testing
A 2-year national survey was initiated in 2011 to identify the status of apple scab pathogen resistance to fungicide in Canadian orchards. In total, 98 orchard sites across Canada (Table 1) were tested for resistance to the SI fungicides (Nova 40W and Inspire) and the strobilurin fungicide (Flint 50 WG).
Several apple trees were left unsprayed for diseases in commercial orchards of grower cooperators. From these trees, samples of fresh primary scab lesions were collected between May and June each year and were sent to the University of Guelph’s Pest Diagnostic Laboratory for testing. The primary resistance test was a modification on a monoconidial isolation (SMOR) method developed at Cornell University. Conidia were collected from symptomatic leaf tissues and incubated on an agar plate for 1-2 days. Germinating conidia were transferred to new plates to form pure colonies of isolates.
Isolates were plated on media treated with either Nova/myclobutanil, Inspire/difenoconazole, or Flint/trifloxystrobin fungicide and allowed to grow for 14 days. Relative growth was determined by measuring the diameter of isolates in each fungicide treated plate on day 14 and comparing it to the untreated control. Figures 3 and 4 depict the typical growth patterns observed in a susceptible and resistant apple scab population, respectively.

Image showing five Petri plates where susceptible apple scab isolates are grown in media treated with each of the four test fungicides (Myclobutanil, Difenoconazole, Trifloxystrobin, and Trifloxystrobin SHAM) and an untreated control. The sizes of isolate growth in fungicide-treated media are significantly smaller than isolates grown in the control plate.
Figure 3: Susceptible pathogen: apple scab isolates grown in fungicide-treated media are significantly smaller than isolates grown in an untreated control plate.
Photo credit: Melody Melzer, University of Guelph’s Pest Diagnostic Lab

Figure 4: Resistant pathogen: apple scab isolates grown in Nova (myclobutanil)- and Flint (trifloxystrobin)-treated media are similar in diameter to isolates grown in the control plate.
Photo credit: Melody Melzer, University of Guelph’s Pest Diagnostic Lab
Results of the growth bioassays were interpreted using relative growth thresholds set to determine the sensitivity levels of pathogen isolates to SI or strobilurin fungicides. The pathogen populations were classified into three categories:
- Sensitive, if the relative growth was below the susceptible baseline;
- Shifting towards resistance, if the relative growth was between the susceptible baseline and the resistance threshold; and
- Resistant, if the relative growth was above the resistance threshold.
An additional test for resistance, developed by Michigan State University, was used to determine the presence of the G143A mutation, which indicates a high level of resistance to strobilurins. The presence of the G143A fragment was determined by using PCR in 10 isolates per orchard in 74 orchards out of the 98 that participated in the survey.
Results
Sterol inhibitor fungicides
The results for Nova indicate that the populations tested were shifting, or had shifted, from susceptible to resistant
(Figure 5). The only susceptible sites were two wild, unsprayed orchards (one in Ontario and one in Nova Scotia). However, Nova may still be useful for powdery mildew control.
For Inspire, the results indicate that the majority of the populations tested are still susceptible (Figure 6). However, approx. 50% of the samples are above 30% relative growth and 20% of the populations are in the shifting phase. Although Nova and Inspire are in the same chemical family, Inspire belongs to the second generation with greater efficacy compared to the first generation of SI’s. This fungicide has only been registered in Canada since 2011 and has had limited use to date. However, selection for pathogen resistance to this fungicide may be fairly rapid in some orchards, given that resistance to this chemical class has been established with Nova.
Figure 5: Sensitivity of apple scab populations to Nova fungicide in each province, 2011-2012.
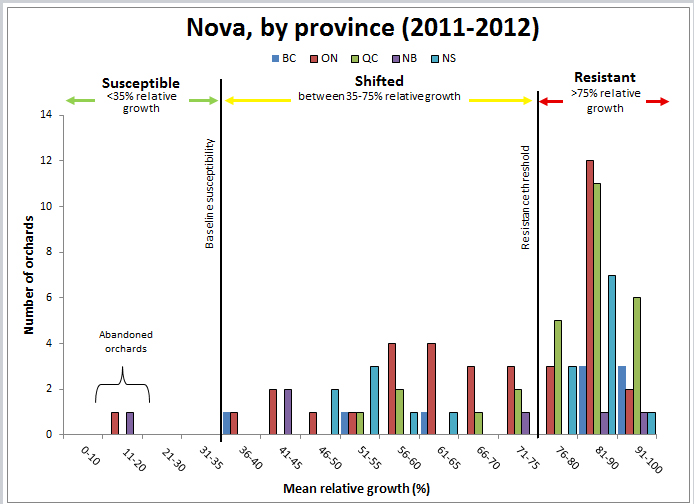
The table below represents the data to produce the graph above
| Mean relative growth (percent) by province | 0-10 | 11-20 | 21-30 | 31-35 | 36-40 | 41-45 | 46-50 | 51-55 | 56-60 | 61-65 | 66-70 | 71-75 | 76-80 | 81-90 | 91-100 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| British Columbia | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 3 | 3 | 9 |
| Ontario | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 1 | 4 | 4 | 3 | 3 | 3 | 12 | 2 | 37 |
| Quebec | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 2 | 5 | 11 | 6 | 28 |
| New Brunswick | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 6 |
| Nova Scotia | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 1 | 1 | 0 | 0 | 3 | 7 | 1 | 18 |
Figure 6: Sensitivity of apple scab populations to Inspire fungicide in each province, 2011-2012.
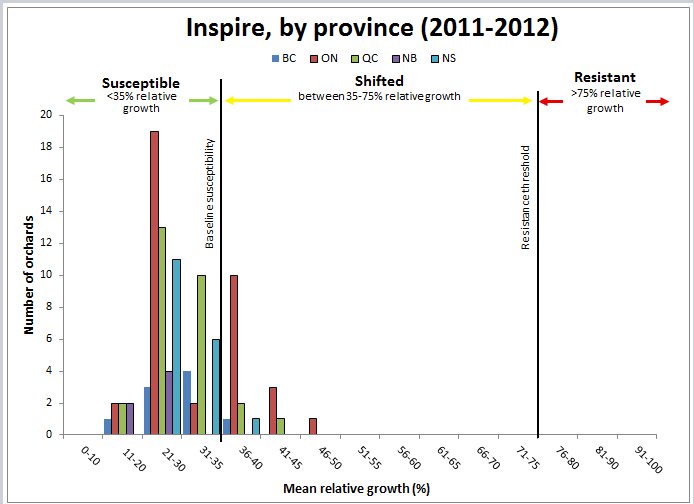
The table below represents the data to produce the graph above
| Mean relative growth (percent)by province | 0-10 | 11-20 | 21-30 | 31-35 | 36-40 | 41-45 | 46-50 | 51-55 | 56-60 | 61-65 | 66-70 | 71-75 | 76-80 | 81-90 | 91-100 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| British Columbia | 0 | 1 | 3 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 |
| Ontario | 0 | 2 | 19 | 2 | 10 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 37 |
| Quebec | 0 | 2 | 13 | 10 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 28 |
| New Brunswick | 0 | 2 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| Nova Scotia | 0 | 0 | 11 | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 18 |
Figure 7: Sensitivity of apple scab populations to Flint fungicide in each province, 2011-2012.
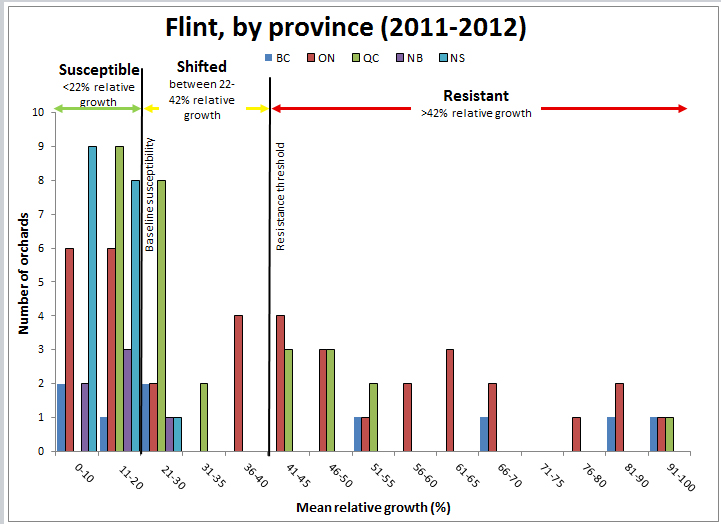
The table below represents the data to produce the graph above
| Mean relative growth (percent) by province | 0-10 | 11-20 | 21-30 | 31-35 | 36-40 | 41-45 | 46-50 | 51-55 | 56-60 | 61-65 | 66-70 | 71-75 | 76-80 | 81-90 | 91-100 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| British Columbia | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 9 |
| Ontario | 6 | 6 | 2 | 0 | 4 | 4 | 3 | 1 | 2 | 3 | 2 | 0 | 1 | 2 | 1 | 37 |
| Quebec | 0 | 9 | 8 | 2 | 0 | 3 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 28 |
| New Brunswick | 2 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| Nova Scotia | 9 | 8 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 18 |
Strobilurin fungicides
For Flint, the distribution of resistance among isolates ranged from susceptible to resistant (Figure 7). Strobilurins have not been used for as long as, or as extensively as, the SI fungicides in some regions which could explain the variability among provinces. All orchards that were classified as Shifted or Resistant to Flint also tested positive for the G143A mutation (Table 1), which would confer resistance to all strobilurin fungicides.
| Province | Number of orchards sampled - Growth bioassay | Number of orchards sampled - DNA Screening | Presence of G143A mutation - Number of positive orchards | Presence of G143A mutation - Percent positive isolates per orchard |
|---|---|---|---|---|
| British Columbia | 11 | 6 | 1 | 100 |
| Ontario | 37 | 31 | 17 | 10-100 |
| Quebec | 26 | 22 | 10 | 10-100 |
| New Brunswick | 6 | 2 | 0 | 0 |
| Nova Scotia | 18 | 14 | 1 | 10 |
| Total | 98 | 75 | 29 | 10-100 |
Resistance Management
The likelihood of the pathogen to develop resistance to fungicides, and the success of fungicide applications to control the disease, are affected by various factors. These include the type of fungicide used (for example broad-spectrum with multi-site activity are preferred over single-site), timing of application (for example preventative and in accordance with local conditions are preferred over post-infection or by calendar), application regime (for example no more than 2 back-to-back sprays of a fungicide group with a site-specific mode of action), sanitation, and others.
To improve decision making, pathogen samples taken from individual orchards need to be tested for resistance and each product needs to be evaluated. If an orchard is found to contain a resistant pathogen, chemical control will eventually fail if the use of the specific product continues. Testing the pathogen for resistance to fungicides is challenging, expensive, and, at times, inconclusive; however, it can be a useful tool to make appropriate control strategy decisions.
As a result of this project, the University of Guelph’s Pest Diagnostics Laboratory has developed a standard protocol to test for apple scab pathogen resistance to fungicides. The lab now offers the diagnostic service to growers for a fee. This service enables growers and industry to test the status of pathogen resistance to a range of fungicides, including SIs, strobilurins, and other products, such as dodine. This new tool allows for better decisions regarding the use of fungicides, including tank mixes, appropriate timing and number of applications. It could prolong the longevity of registered products or, if resistance is detected in an orchard, it could help keep the use of these chemicals to a minimum.
Additional practices for resistance management, such as sanitation, are included in a guide published under the Apple Scab strategy. The guide contains information about management of primary and secondary infections and is available Apple scab: Improving understanding for better management.
For more information about this project, please contact:
Kelly Ciceran, General Manager
Ontario Apple Growers
P.O. Box 100
Vineland Station, ON L0R 2E0
Phone: 905-688-0990 ext. 241
Fax: 905-688-5915
Email: kciceran@onapples.com
Acknowledgements
This work was in partnership with Ontario Apple Growers, Apple Growers of New Brunswick, Nova Scotia Fruit Growers’ Association, Fédération des producteurs de pommes du Québec, British Columbia Fruit Growers’ Association and the Apple Working Group of the Canadian Horticulture Council.
The authors acknowledge the collaboration of Melody Melzer, Shannon Xuechan Shan, Todd Marrow and Gabe Ho at the University of Guelph’s Pest Diagnostic Laboratory; Dr. Kerik Cox, Cornell University; and Dr. George Sundin, Michigan State University. In addition to Agriculture and Agri-Food Canada’s Pest Management Centre, funding has been provided by Dow AgroSciences Canada, Syngenta Crop Protection, E.I. DuPont Canada, BASF Canada Inc., and Bayer CropScience Inc.
Other useful resources
For region-specific resistance management recommendations refer to your local field person or extension specialist.
Additional resources can be found at:
- British Columbia
Apple Scab Management in British Columbia - Ontario
Information for Commercial Apple Growers in Ontario - Quebec
Arbres fruitiers (in French only) - Nova Scotia
Managing Apple Scab Resistance in Nova Scotia Orchards (PDF)
About the Pesticide Risk Reduction at Agriculture and Agri-Food Canada
The Pesticide Risk Reduction team delivers viable solutions for Canadian growers to reduce pesticide risks in the agricultural and agri-food industry. The team achieves this goal by funding integrated pest management projects and coordinating pesticide risk reduction strategies developed through consultation with stakeholders and pest management experts. Other sustainable crop protection factsheets are available. For more information please visit the Pest Management Centre.